Key Insights
The France respiratory devices market, valued at approximately €[Estimate based on market size XX and currency conversion, e.g., €250 million] in 2025, is projected to experience robust growth, exhibiting a Compound Annual Growth Rate (CAGR) of 6.50% from 2025 to 2033. This expansion is fueled by several key factors. The increasing prevalence of chronic respiratory illnesses like asthma, COPD, and cystic fibrosis, coupled with an aging population, significantly contributes to the demand for respiratory devices. Technological advancements leading to the development of smaller, more portable, and user-friendly devices are also driving market growth. Furthermore, rising healthcare expenditure and improving healthcare infrastructure in France are facilitating wider adoption of respiratory therapies. The market is segmented by type, encompassing diagnostic and monitoring devices (such as spirometers, oximeters, and peak flow meters) and therapeutic devices (including inhalers, ventilators, and nebulizers), with disposables forming a significant sub-segment. Competition within the market is intense, with major players like Fisher & Paykel Healthcare, Drägerwerk, Invacare, GSK, and ResMed vying for market share. While the market enjoys considerable potential, challenges exist, including high costs associated with advanced devices and potential reimbursement hurdles.
The market's future trajectory hinges on several crucial trends. The growing adoption of telehealth and remote patient monitoring solutions is expected to enhance accessibility and efficiency of respiratory care. The ongoing research and development efforts focused on innovative device technologies, particularly those employing AI and connected health features, will likely shape the market landscape in the coming years. Government initiatives promoting preventative healthcare and early diagnosis of respiratory ailments will further augment market growth. However, potential restraints include stringent regulatory requirements for medical devices and the risk of generic competition impacting pricing dynamics. Overall, the France respiratory devices market presents a promising investment opportunity for stakeholders, supported by a confluence of positive drivers and technological innovations, despite certain inherent challenges.
France Respiratory Devices Market: A Comprehensive Report (2019-2033)
This dynamic report provides a detailed analysis of the France respiratory devices market, offering invaluable insights for stakeholders across the medical technology industry. Leveraging extensive research and data spanning the period from 2019 to 2033, this report is a crucial resource for understanding market trends, competitive dynamics, and future growth opportunities. The report projects a market size of xx Million by 2025 and reveals a significant CAGR of xx% during the forecast period (2025-2033).
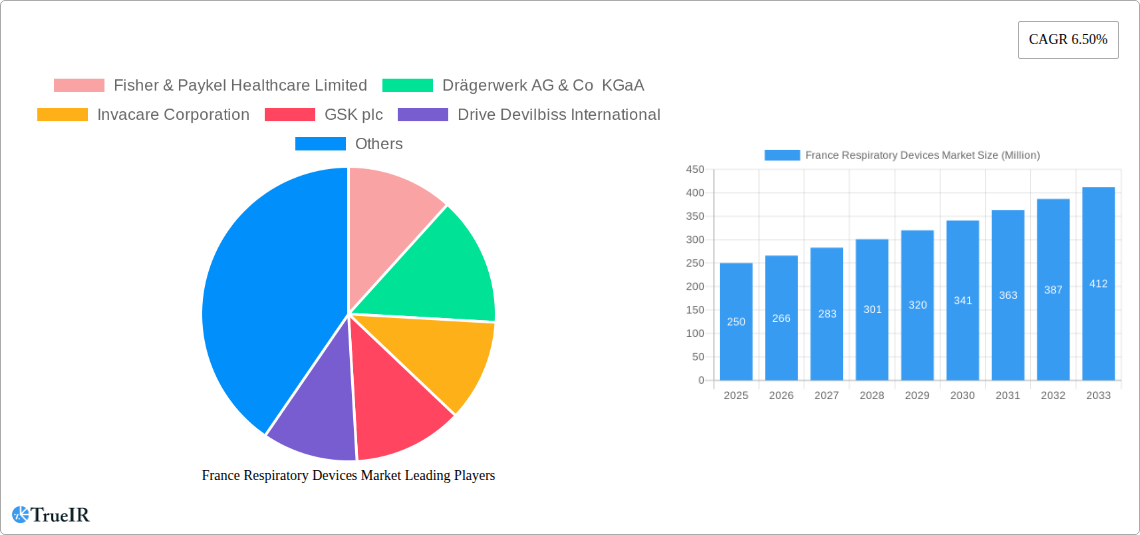
France Respiratory Devices Market Market Structure & Competitive Landscape
The France respiratory devices market exhibits a moderately consolidated structure, with key players such as Fisher & Paykel Healthcare Limited, Drägerwerk AG & Co KGaA, Invacare Corporation, GSK plc, Drive Devilbiss International, GE Healthcare, Koninklijke Philips N.V., ResMed Inc., and Medtronic plc holding significant market share. However, the presence of numerous smaller players and emerging innovative companies indicates a competitive landscape characterized by both established giants and agile newcomers.
Market Concentration: The four-firm concentration ratio (CR4) is estimated at xx%, suggesting a moderately consolidated market with room for smaller players to thrive.
Innovation Drivers: Technological advancements in areas such as telemonitoring, AI-driven diagnostics, and minimally invasive therapies are crucial innovation drivers. The increasing prevalence of chronic respiratory diseases and the growing demand for home healthcare solutions fuel further innovation.
Regulatory Impacts: Stringent regulatory frameworks concerning medical device approvals and safety standards in France significantly impact market dynamics. Compliance requirements and reimbursement policies shape product development and market entry strategies.
Product Substitutes: While direct substitutes are limited, certain therapies and alternative treatment approaches present indirect competition. The market observes a push towards minimally invasive and less disruptive procedures.
End-User Segmentation: The market caters primarily to hospitals, clinics, and home healthcare settings. The growing preference for home-based respiratory care drives segment growth, with projections of xx Million in revenue by 2033.
M&A Trends: The sector witnessed xx M&A transactions between 2019 and 2024, indicating a moderate level of consolidation. These activities primarily involved strategic acquisitions of smaller companies with specialized technologies or strong market positions. The trend is expected to continue with an estimated xx more acquisitions in the forecast period driven by both established players and private equity firms seeking market consolidation.
France Respiratory Devices Market Market Trends & Opportunities
The France respiratory devices market is experiencing robust growth fueled by several key trends. The aging population, the rising prevalence of chronic respiratory diseases (e.g., asthma, COPD, cystic fibrosis), and increasing healthcare expenditure contribute to market expansion. Technological advancements, such as the integration of digital health technologies and the development of advanced diagnostic and therapeutic devices, are driving market transformation. Consumer preferences shift towards convenient, user-friendly devices suitable for home-based care, stimulating demand for telehealth solutions and remote patient monitoring systems. The market is witnessing an increase in adoption of advanced therapies (e.g., non-invasive ventilation) and connected devices for better patient care. Furthermore, evolving reimbursement policies and government initiatives promoting preventative care drive growth. The competitive dynamics are shaped by both established multinational corporations and innovative startups vying for market share. The market is poised for growth, projecting xx Million by 2033 with a CAGR of xx%.
Dominant Markets & Segments in France Respiratory Devices Market
The French respiratory devices market demonstrates robust growth across various segments and regions. The therapeutic devices segment is expected to dominate, owing to increasing demand for effective respiratory treatments.
By Type:
- Diagnostic and Monitoring Devices: This segment holds a significant share, driven by the increasing need for early diagnosis and ongoing monitoring of respiratory conditions. Technological advancements like portable spirometers and home-based pulse oximeters contribute to its growth. The projected market size for this segment is xx Million by 2033.
- Therapeutic Devices: This segment commands the largest share due to the high prevalence of chronic respiratory diseases requiring continuous treatment. Advanced ventilation systems and oxygen therapy equipment drive this segment’s strong performance. The projected market size for this segment is xx Million by 2033.
- Disposables: The disposables segment shows steady growth driven by the need for single-use items like oxygen cannulas and nebulizer masks. The segment's growth is closely tied to the overall increase in respiratory device usage. The projected market size for this segment is xx Million by 2033.
Key Growth Drivers:
- Strong healthcare infrastructure: France possesses a well-developed healthcare system supporting extensive diagnostics and therapeutic capabilities.
- Favorable government policies: Government initiatives aimed at improving respiratory health and expanding access to healthcare services create a positive market environment.
- Technological advancements: Continuous innovation in respiratory devices contributes to enhanced treatment efficacy and patient comfort.
France Respiratory Devices Market Product Analysis
The market features a wide array of products, ranging from basic diagnostic tools like spirometers to advanced ventilation systems and telemonitoring devices. Technological advancements emphasize miniaturization, improved portability, and seamless integration with digital health platforms. Products are tailored to meet diverse patient needs, with a growing focus on home-based solutions that enhance patient comfort and convenience while reducing hospital readmissions. Competition hinges on factors like technological superiority, user-friendliness, and cost-effectiveness.
Key Drivers, Barriers & Challenges in France Respiratory Devices Market
Key Drivers:
The market is driven by the increasing prevalence of respiratory illnesses, technological advancements such as smart inhalers and wearable sensors, growing demand for home-based respiratory care, and supportive government policies promoting access to healthcare.
Challenges and Restraints:
High costs associated with advanced respiratory devices limit accessibility, particularly for patients in low-income groups. Stringent regulatory approvals and reimbursement processes pose challenges for market entry and growth, particularly for smaller companies. Intense competition from established players further adds to the challenges. The market is also vulnerable to supply chain disruptions and the impact of economic downturns.
Growth Drivers in the France Respiratory Devices Market Market
The market is driven by factors like an aging population, increasing prevalence of respiratory diseases, technological advancements in areas such as telemonitoring, and a rise in the demand for home-based respiratory care. Furthermore, supportive government initiatives, focused on improving respiratory healthcare access and patient outcomes, are also significant contributors to market growth.
Challenges Impacting France Respiratory Devices Market Growth
Challenges include high device costs limiting accessibility for certain demographics, stringent regulatory procedures creating market entry barriers, and intensive competition among established and emerging players. Supply chain vulnerabilities and economic fluctuations also pose significant risks.
Key Players Shaping the France Respiratory Devices Market Market
- Fisher & Paykel Healthcare Limited
- Drägerwerk AG & Co KGaA
- Invacare Corporation
- GSK plc
- Drive Devilbiss International
- GE Healthcare
- Koninklijke Philips N.V.
- ResMed Inc.
- Medtronic plc
Significant France Respiratory Devices Market Industry Milestones
- November 2022: Biosency partnered with ResMed to distribute Bora-Care, a telemonitoring device for homecare respiratory patients, significantly boosting the home healthcare segment.
- October 2022: Medtronic's plan to separate its Patient Monitoring and Respiratory Interventions business is expected to reshape the competitive landscape within the next 12-18 months.
Future Outlook for France Respiratory Devices Market Market
The France respiratory devices market is poised for continued growth, driven by sustained technological advancements, rising prevalence of chronic respiratory diseases, and a growing demand for home-based respiratory care. Strategic partnerships and investments in innovative technologies will further propel market expansion. The market’s strong future hinges on continued innovation, addressing accessibility challenges, and navigating regulatory complexities.
France Respiratory Devices Market Segmentation
-
1. Type
-
1.1. Diagnostic and Monitoring Devices
- 1.1.1. Spirometers
- 1.1.2. Sleep Test Devices
- 1.1.3. Peak Flow Meters
- 1.1.4. Pulse Oximeters
- 1.1.5. Capnographs
- 1.1.6. Other Diagnostic and Monitoring Devices
-
1.2. Therapeutic Devices
- 1.2.1. CPAP Devices
- 1.2.2. BiPAP Devices
- 1.2.3. Humidifiers
- 1.2.4. Nebulizers
- 1.2.5. Oxygen Concentrators
- 1.2.6. Ventilators
- 1.2.7. Inhalers
- 1.2.8. Other Therapeutic Devices
-
1.3. Disposables
- 1.3.1. Masks
- 1.3.2. Breathing Circuits
- 1.3.3. Other Disposables
-
1.1. Diagnostic and Monitoring Devices
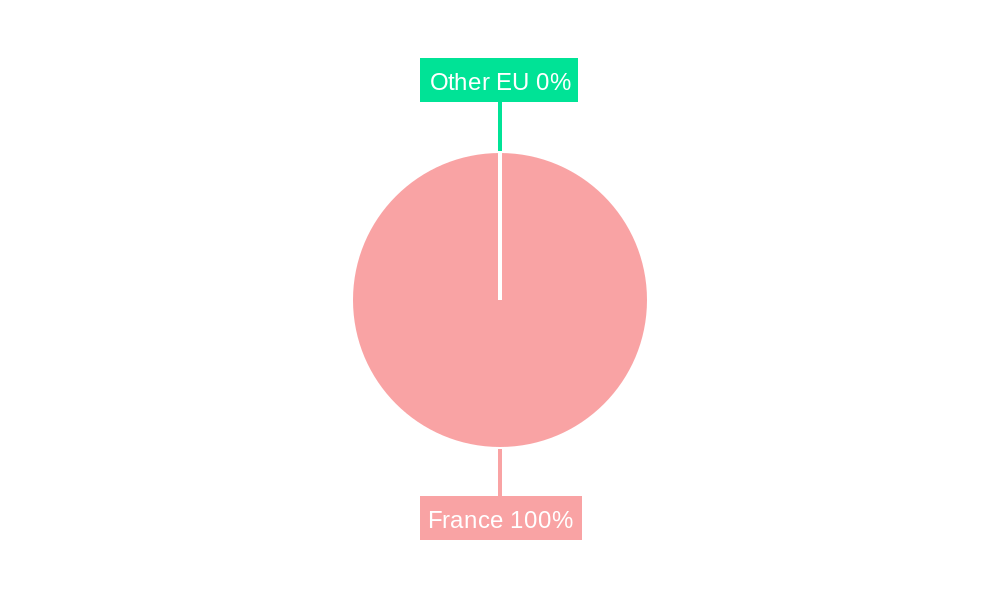
France Respiratory Devices Market Segmentation By Geography
- 1. France
France Respiratory Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Respiratory Disorders; Technological Advancements
- 3.3. Market Restrains
- 3.3.1. High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Spirometers Segment is Expected to Have Significant Growth in the Market Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. France Respiratory Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Diagnostic and Monitoring Devices
- 5.1.1.1. Spirometers
- 5.1.1.2. Sleep Test Devices
- 5.1.1.3. Peak Flow Meters
- 5.1.1.4. Pulse Oximeters
- 5.1.1.5. Capnographs
- 5.1.1.6. Other Diagnostic and Monitoring Devices
- 5.1.2. Therapeutic Devices
- 5.1.2.1. CPAP Devices
- 5.1.2.2. BiPAP Devices
- 5.1.2.3. Humidifiers
- 5.1.2.4. Nebulizers
- 5.1.2.5. Oxygen Concentrators
- 5.1.2.6. Ventilators
- 5.1.2.7. Inhalers
- 5.1.2.8. Other Therapeutic Devices
- 5.1.3. Disposables
- 5.1.3.1. Masks
- 5.1.3.2. Breathing Circuits
- 5.1.3.3. Other Disposables
- 5.1.1. Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. France
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2024
- 6.2. Company Profiles
- 6.2.1 Fisher & Paykel Healthcare Limited
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Drägerwerk AG & Co KGaA
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Invacare Corporation
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 GSK plc
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Drive Devilbiss International
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 GE Healthcare
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Koninklijke Philips N V
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 ResMed Inc*List Not Exhaustive
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Medtronic plc
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.1 Fisher & Paykel Healthcare Limited
List of Figures
- Figure 1: France Respiratory Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: France Respiratory Devices Market Share (%) by Company 2024
List of Tables
- Table 1: France Respiratory Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: France Respiratory Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 3: France Respiratory Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 4: France Respiratory Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 5: France Respiratory Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 6: France Respiratory Devices Market Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the France Respiratory Devices Market?
The projected CAGR is approximately 6.50%.
2. Which companies are prominent players in the France Respiratory Devices Market?
Key companies in the market include Fisher & Paykel Healthcare Limited, Drägerwerk AG & Co KGaA, Invacare Corporation, GSK plc, Drive Devilbiss International, GE Healthcare, Koninklijke Philips N V, ResMed Inc*List Not Exhaustive, Medtronic plc.
3. What are the main segments of the France Respiratory Devices Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders; Technological Advancements.
6. What are the notable trends driving market growth?
Spirometers Segment is Expected to Have Significant Growth in the Market Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Devices.
8. Can you provide examples of recent developments in the market?
November 2022: Biosency entered into a strategic partnership with ResMed. Under this collaboration, ResMed will distribute BIOSENCY's telemonitoring device, Bora-Care, in France. This device is aimed at supporting homecare patients dealing with respiratory insufficiency.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "France Respiratory Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the France Respiratory Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the France Respiratory Devices Market?
To stay informed about further developments, trends, and reports in the France Respiratory Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


