Key Insights
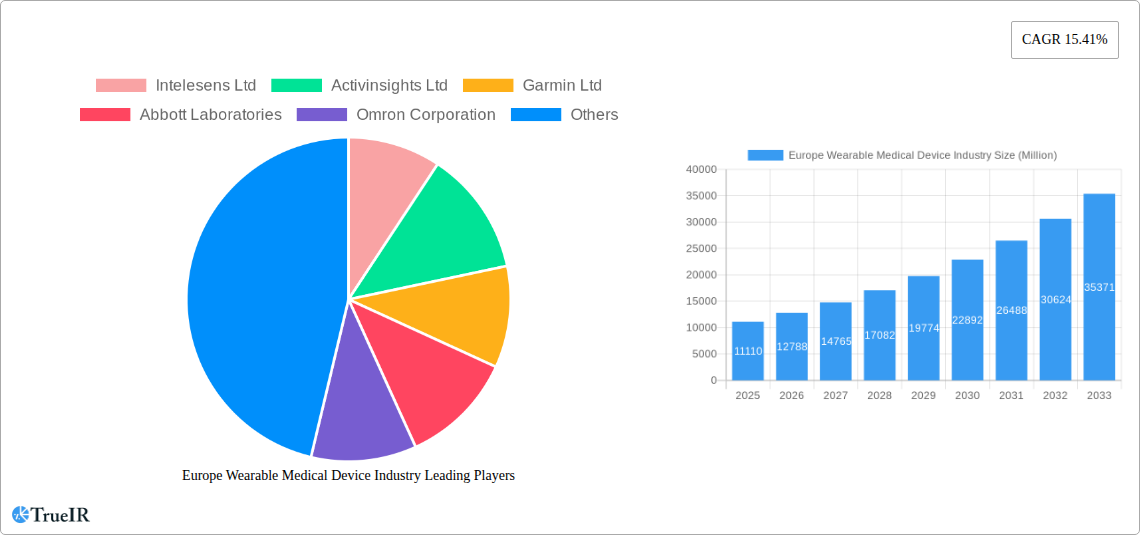
The European wearable medical device market, valued at €11.11 billion in 2025, is projected to experience robust growth, exhibiting a Compound Annual Growth Rate (CAGR) of 15.41% from 2025 to 2033. This expansion is fueled by several key factors. The increasing prevalence of chronic diseases like diabetes and cardiovascular conditions, coupled with a rising geriatric population requiring remote health monitoring, significantly drives demand. Technological advancements leading to smaller, more comfortable, and feature-rich devices further enhance market appeal. The integration of wearable devices with smartphone applications and cloud-based platforms facilitates seamless data transmission and analysis, enabling proactive healthcare interventions and improved patient outcomes. Specifically, segments like remote patient monitoring and home healthcare are witnessing exponential growth, driven by the need for cost-effective and convenient healthcare solutions. Strong government initiatives promoting telehealth and digital health also contribute to the market's upward trajectory. Germany, the United Kingdom, and France are major contributors to the European market share, reflecting their advanced healthcare infrastructure and adoption of innovative technologies.
Competition in the European market is intense, with established players like Garmin, Abbott Laboratories, and Philips competing alongside innovative startups such as Intelesens and Activinsights. While the market presents significant opportunities, challenges remain. Data privacy concerns, regulatory hurdles related to medical device approvals, and the need for robust cybersecurity measures represent potential restraints. However, ongoing research and development efforts focused on improving device accuracy, battery life, and user experience are expected to mitigate these challenges and further stimulate market growth. The diversification of product types— encompassing watches, wristbands, ear wear, and other monitoring devices—offers opportunities for market players to cater to a wider range of patient needs and preferences. The focus will likely remain on providing accurate, reliable, and user-friendly devices that seamlessly integrate into individuals' daily lives, contributing to improved health management and better patient outcomes.
Europe Wearable Medical Device Industry: Market Report 2019-2033
This comprehensive report provides an in-depth analysis of the European wearable medical device industry, offering crucial insights for investors, manufacturers, and stakeholders. Covering the period 2019-2033, with a base year of 2025, this report analyzes market trends, competitive dynamics, and future growth potential, projecting a market valued at xx Million by 2033.
Europe Wearable Medical Device Industry Market Structure & Competitive Landscape
The European wearable medical device market exhibits a moderately concentrated structure, with a few major players holding significant market share alongside numerous smaller, specialized companies. Key innovation drivers include advancements in sensor technology, miniaturization, AI integration, and improved data analytics capabilities. Stringent regulatory frameworks, such as the MDR (Medical Device Regulation), significantly impact market entry and product development. Product substitutes, including traditional medical monitoring equipment, pose a challenge, though the convenience and accessibility of wearables continue to drive market growth. End-user segmentation is broad, encompassing individuals focusing on fitness tracking, patients needing remote monitoring, and healthcare providers utilizing the devices for enhanced care. M&A activity is steadily increasing, with xx Million in deals recorded in 2024, indicating consolidation trends within the industry. Concentration ratios (e.g., CR4) are expected to increase slightly to xx% by 2033 as larger companies acquire smaller players to expand their product portfolios and market reach.
- Market Concentration: Moderately concentrated, with a CR4 of xx% in 2024, projected to rise to xx% by 2033.
- Innovation Drivers: Sensor technology, miniaturization, AI, data analytics.
- Regulatory Impacts: Stringent MDR regulations influence market dynamics.
- Product Substitutes: Traditional medical monitoring equipment.
- End-User Segmentation: Fitness enthusiasts, patients, healthcare providers.
- M&A Trends: Increasing consolidation, with xx Million in deals in 2024.
Europe Wearable Medical Device Industry Market Trends & Opportunities
The European wearable medical device market is experiencing robust growth, driven by several key factors. The market size is projected to reach xx Million by 2025, growing at a CAGR of xx% from 2025 to 2033. This growth is fueled by the increasing adoption of connected health solutions, rising health consciousness among consumers, and technological advancements leading to more sophisticated and user-friendly devices. Consumer preferences are shifting towards devices with advanced features, improved accuracy, seamless integration with smartphones, and long battery life. Competitive dynamics are characterized by both intense rivalry among established players and the emergence of innovative start-ups. Market penetration is currently at xx% and is projected to reach xx% by 2033, indicating significant untapped potential for growth across various segments. The rising prevalence of chronic diseases and the increasing demand for remote patient monitoring are further bolstering market growth.
Dominant Markets & Segments in Europe Wearable Medical Device Industry
The dominant segment within the European wearable medical device market varies depending on the categorization.
By Product Type: Smartwatches dominate, holding a market share of approximately xx% in 2025. Wristbands hold the second largest share.
By Device Type: Monitoring devices (heart rate, activity, sleep) are the most prevalent, followed by therapeutic devices such as those designed for rehabilitation and chronic condition management. Neuromonitoring devices are a growing but relatively smaller segment.
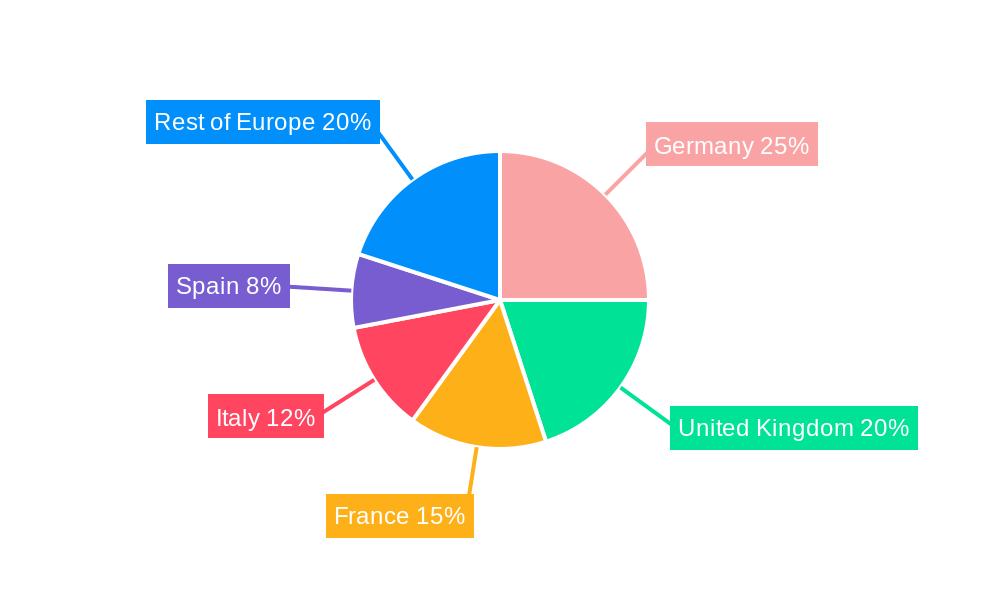
By Application: The sports and fitness segment currently commands the largest share, driven by consumer demand for fitness trackers and smartwatches. However, the remote patient monitoring and home healthcare sectors are showing rapid growth and are expected to capture a significant market share in the coming years. Germany and the UK are the largest national markets.
- Key Growth Drivers (Germany and UK):
- Strong healthcare infrastructure
- Favorable government policies supporting digital health
- High consumer adoption of technology
- Large aging population needing remote healthcare solutions
Europe Wearable Medical Device Industry Product Analysis
The European wearable medical device market showcases a wide array of products, ranging from basic fitness trackers to sophisticated medical-grade devices capable of continuous monitoring of vital signs. Recent innovations focus on improved sensor technology (e.g., optical heart rate sensors, ECG), enhanced data analytics using AI and machine learning, and better integration with healthcare platforms for seamless data sharing and remote consultations. The success of new products hinges on their accuracy, ease of use, aesthetic appeal, and integration capabilities. Products featuring advanced features such as fall detection, medication reminders, and real-time health alerts are gaining traction.
Key Drivers, Barriers & Challenges in Europe Wearable Medical Device Industry
Key Drivers:
- Technological advancements in miniaturization, sensor technology, and AI.
- Increasing adoption of telehealth and remote patient monitoring.
- Rising prevalence of chronic diseases requiring continuous health monitoring.
- Favorable regulatory environment in certain European countries.
Challenges:
- Stringent regulatory hurdles and approval processes for medical devices (MDR).
- Data privacy and security concerns regarding sensitive health data.
- Competition from established players and new entrants.
- Supply chain disruptions impacting the availability of components.
- Concerns about the accuracy and reliability of data from some wearable devices.
Growth Drivers in the Europe Wearable Medical Device Industry Market
Growth is driven by technological advancements, the increasing prevalence of chronic diseases, rising adoption of telehealth, and favorable government policies supporting digital healthcare initiatives. Miniaturization of components, improved sensor technology, and AI integration are enhancing device capabilities and usability.
Challenges Impacting Europe Wearable Medical Device Industry Growth
Challenges include stringent regulatory pathways (MDR compliance), data privacy and security concerns, supply chain vulnerabilities, and intense competition. These factors impact product development timelines, costs, and market entry strategies.
Key Players Shaping the Europe Wearable Medical Device Industry Market
- Intelesens Ltd
- Activinsights Ltd
- Garmin Ltd
- Abbott Laboratories
- Omron Corporation
- Polar Electro Oy
- Fitbit Inc
- Nuubo
- Koninklijke Philips NV
Significant Europe Wearable Medical Device Industry Industry Milestones
- March 2022: Infineon Technologies AG and Sleepiz AG launched Infineon XENSIV 60 GHz radar technology for contactless vital sign monitoring. This technology significantly enhances the capabilities of smart home and healthcare devices.
- February 2022: Oppo launched the Oppo Watch Free smartwatch in the European market, featuring extensive sports tracking and health monitoring capabilities. This launch indicates expanding market reach for wearable devices.
Future Outlook for Europe Wearable Medical Device Industry Market
The European wearable medical device market is poised for continued strong growth, driven by technological innovation, increasing healthcare spending, and the growing demand for remote patient monitoring solutions. The integration of AI and machine learning will further enhance device capabilities and data analysis, leading to improved diagnostics and personalized healthcare. New market opportunities lie in developing wearables for specific chronic conditions, integrating devices with electronic health records (EHRs), and strengthening data security and privacy measures.
Europe Wearable Medical Device Industry Segmentation
-
1. Device Type
-
1.1. Monitoring Devices
- 1.1.1. Vital Sign Monitoring Devices
- 1.1.2. Sleep Monitoring Devices
- 1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 1.1.4. Neuromonitoring Devices
-
1.2. Therapeutic Devices
- 1.2.1. Pain Management Devices
- 1.2.2. Rehabilitation Devices
- 1.2.3. Respiratory Therapy Devices
- 1.2.4. Other Theraputic Devices
-
1.1. Monitoring Devices
-
2. Application
- 2.1. Sports and Fitness
- 2.2. Remote Patient Monitoring
- 2.3. Home Healthcare
-
3. Product Type
- 3.1. Watch
- 3.2. Wristband
- 3.3. Ear Wear
- 3.4. Other Product Types
Europe Wearable Medical Device Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Wearable Medical Device Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 15.41% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Technological Advancements and Innovation; Increasing Health Awareness; Ease of Use and Interpretation of Data
- 3.3. Market Restrains
- 3.3.1. Lack of Reimbursement Policies
- 3.4. Market Trends
- 3.4.1. Remote Patient Monitoring Segment is Expected to Grow Rapidly Over the Forecast Period in the Europe Wearable Medical Devices Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Monitoring Devices
- 5.1.1.1. Vital Sign Monitoring Devices
- 5.1.1.2. Sleep Monitoring Devices
- 5.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 5.1.1.4. Neuromonitoring Devices
- 5.1.2. Therapeutic Devices
- 5.1.2.1. Pain Management Devices
- 5.1.2.2. Rehabilitation Devices
- 5.1.2.3. Respiratory Therapy Devices
- 5.1.2.4. Other Theraputic Devices
- 5.1.1. Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Sports and Fitness
- 5.2.2. Remote Patient Monitoring
- 5.2.3. Home Healthcare
- 5.3. Market Analysis, Insights and Forecast - by Product Type
- 5.3.1. Watch
- 5.3.2. Wristband
- 5.3.3. Ear Wear
- 5.3.4. Other Product Types
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Germany
- 5.4.2. United Kingdom
- 5.4.3. France
- 5.4.4. Italy
- 5.4.5. Spain
- 5.4.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Germany Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Monitoring Devices
- 6.1.1.1. Vital Sign Monitoring Devices
- 6.1.1.2. Sleep Monitoring Devices
- 6.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 6.1.1.4. Neuromonitoring Devices
- 6.1.2. Therapeutic Devices
- 6.1.2.1. Pain Management Devices
- 6.1.2.2. Rehabilitation Devices
- 6.1.2.3. Respiratory Therapy Devices
- 6.1.2.4. Other Theraputic Devices
- 6.1.1. Monitoring Devices
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Sports and Fitness
- 6.2.2. Remote Patient Monitoring
- 6.2.3. Home Healthcare
- 6.3. Market Analysis, Insights and Forecast - by Product Type
- 6.3.1. Watch
- 6.3.2. Wristband
- 6.3.3. Ear Wear
- 6.3.4. Other Product Types
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. United Kingdom Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Monitoring Devices
- 7.1.1.1. Vital Sign Monitoring Devices
- 7.1.1.2. Sleep Monitoring Devices
- 7.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 7.1.1.4. Neuromonitoring Devices
- 7.1.2. Therapeutic Devices
- 7.1.2.1. Pain Management Devices
- 7.1.2.2. Rehabilitation Devices
- 7.1.2.3. Respiratory Therapy Devices
- 7.1.2.4. Other Theraputic Devices
- 7.1.1. Monitoring Devices
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Sports and Fitness
- 7.2.2. Remote Patient Monitoring
- 7.2.3. Home Healthcare
- 7.3. Market Analysis, Insights and Forecast - by Product Type
- 7.3.1. Watch
- 7.3.2. Wristband
- 7.3.3. Ear Wear
- 7.3.4. Other Product Types
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. France Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Monitoring Devices
- 8.1.1.1. Vital Sign Monitoring Devices
- 8.1.1.2. Sleep Monitoring Devices
- 8.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 8.1.1.4. Neuromonitoring Devices
- 8.1.2. Therapeutic Devices
- 8.1.2.1. Pain Management Devices
- 8.1.2.2. Rehabilitation Devices
- 8.1.2.3. Respiratory Therapy Devices
- 8.1.2.4. Other Theraputic Devices
- 8.1.1. Monitoring Devices
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Sports and Fitness
- 8.2.2. Remote Patient Monitoring
- 8.2.3. Home Healthcare
- 8.3. Market Analysis, Insights and Forecast - by Product Type
- 8.3.1. Watch
- 8.3.2. Wristband
- 8.3.3. Ear Wear
- 8.3.4. Other Product Types
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Italy Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Monitoring Devices
- 9.1.1.1. Vital Sign Monitoring Devices
- 9.1.1.2. Sleep Monitoring Devices
- 9.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 9.1.1.4. Neuromonitoring Devices
- 9.1.2. Therapeutic Devices
- 9.1.2.1. Pain Management Devices
- 9.1.2.2. Rehabilitation Devices
- 9.1.2.3. Respiratory Therapy Devices
- 9.1.2.4. Other Theraputic Devices
- 9.1.1. Monitoring Devices
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Sports and Fitness
- 9.2.2. Remote Patient Monitoring
- 9.2.3. Home Healthcare
- 9.3. Market Analysis, Insights and Forecast - by Product Type
- 9.3.1. Watch
- 9.3.2. Wristband
- 9.3.3. Ear Wear
- 9.3.4. Other Product Types
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. Spain Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Monitoring Devices
- 10.1.1.1. Vital Sign Monitoring Devices
- 10.1.1.2. Sleep Monitoring Devices
- 10.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 10.1.1.4. Neuromonitoring Devices
- 10.1.2. Therapeutic Devices
- 10.1.2.1. Pain Management Devices
- 10.1.2.2. Rehabilitation Devices
- 10.1.2.3. Respiratory Therapy Devices
- 10.1.2.4. Other Theraputic Devices
- 10.1.1. Monitoring Devices
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Sports and Fitness
- 10.2.2. Remote Patient Monitoring
- 10.2.3. Home Healthcare
- 10.3. Market Analysis, Insights and Forecast - by Product Type
- 10.3.1. Watch
- 10.3.2. Wristband
- 10.3.3. Ear Wear
- 10.3.4. Other Product Types
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. Rest of Europe Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 11.1.1. Monitoring Devices
- 11.1.1.1. Vital Sign Monitoring Devices
- 11.1.1.2. Sleep Monitoring Devices
- 11.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 11.1.1.4. Neuromonitoring Devices
- 11.1.2. Therapeutic Devices
- 11.1.2.1. Pain Management Devices
- 11.1.2.2. Rehabilitation Devices
- 11.1.2.3. Respiratory Therapy Devices
- 11.1.2.4. Other Theraputic Devices
- 11.1.1. Monitoring Devices
- 11.2. Market Analysis, Insights and Forecast - by Application
- 11.2.1. Sports and Fitness
- 11.2.2. Remote Patient Monitoring
- 11.2.3. Home Healthcare
- 11.3. Market Analysis, Insights and Forecast - by Product Type
- 11.3.1. Watch
- 11.3.2. Wristband
- 11.3.3. Ear Wear
- 11.3.4. Other Product Types
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 12. Germany Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 13. United Kingdom Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 14. France Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 15. Italy Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 16. Spain Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 17. Rest of Europe Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 18. Competitive Analysis
- 18.1. Market Share Analysis 2024
- 18.2. Company Profiles
- 18.2.1 Intelesens Ltd
- 18.2.1.1. Overview
- 18.2.1.2. Products
- 18.2.1.3. SWOT Analysis
- 18.2.1.4. Recent Developments
- 18.2.1.5. Financials (Based on Availability)
- 18.2.2 Activinsights Ltd
- 18.2.2.1. Overview
- 18.2.2.2. Products
- 18.2.2.3. SWOT Analysis
- 18.2.2.4. Recent Developments
- 18.2.2.5. Financials (Based on Availability)
- 18.2.3 Garmin Ltd
- 18.2.3.1. Overview
- 18.2.3.2. Products
- 18.2.3.3. SWOT Analysis
- 18.2.3.4. Recent Developments
- 18.2.3.5. Financials (Based on Availability)
- 18.2.4 Abbott Laboratories
- 18.2.4.1. Overview
- 18.2.4.2. Products
- 18.2.4.3. SWOT Analysis
- 18.2.4.4. Recent Developments
- 18.2.4.5. Financials (Based on Availability)
- 18.2.5 Omron Corporation
- 18.2.5.1. Overview
- 18.2.5.2. Products
- 18.2.5.3. SWOT Analysis
- 18.2.5.4. Recent Developments
- 18.2.5.5. Financials (Based on Availability)
- 18.2.6 Polar Electro Oy*List Not Exhaustive
- 18.2.6.1. Overview
- 18.2.6.2. Products
- 18.2.6.3. SWOT Analysis
- 18.2.6.4. Recent Developments
- 18.2.6.5. Financials (Based on Availability)
- 18.2.7 Fitbit Inc
- 18.2.7.1. Overview
- 18.2.7.2. Products
- 18.2.7.3. SWOT Analysis
- 18.2.7.4. Recent Developments
- 18.2.7.5. Financials (Based on Availability)
- 18.2.8 Nuubo
- 18.2.8.1. Overview
- 18.2.8.2. Products
- 18.2.8.3. SWOT Analysis
- 18.2.8.4. Recent Developments
- 18.2.8.5. Financials (Based on Availability)
- 18.2.9 Koninklinje Philips NV
- 18.2.9.1. Overview
- 18.2.9.2. Products
- 18.2.9.3. SWOT Analysis
- 18.2.9.4. Recent Developments
- 18.2.9.5. Financials (Based on Availability)
- 18.2.1 Intelesens Ltd
List of Figures
- Figure 1: Europe Wearable Medical Device Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Wearable Medical Device Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Wearable Medical Device Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Wearable Medical Device Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 3: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 4: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 5: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 6: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 7: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 8: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 9: Europe Wearable Medical Device Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 10: Europe Wearable Medical Device Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 11: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 13: Germany Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Germany Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 15: United Kingdom Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: United Kingdom Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 17: France Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: France Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 19: Italy Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Italy Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 21: Spain Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Spain Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 23: Rest of Europe Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: Rest of Europe Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 25: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 26: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 27: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 28: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 29: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 30: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 31: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 32: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 33: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 34: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 35: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 36: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 37: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 38: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 39: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 40: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 41: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 42: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 43: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 44: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 45: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 46: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 47: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 48: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 49: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 50: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 51: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 52: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 53: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 54: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 55: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 56: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 57: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 58: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 59: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 60: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 61: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 62: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 63: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 64: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 65: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 66: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 67: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 68: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 69: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 70: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 71: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 72: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Wearable Medical Device Industry?
The projected CAGR is approximately 15.41%.
2. Which companies are prominent players in the Europe Wearable Medical Device Industry?
Key companies in the market include Intelesens Ltd, Activinsights Ltd, Garmin Ltd, Abbott Laboratories, Omron Corporation, Polar Electro Oy*List Not Exhaustive, Fitbit Inc, Nuubo, Koninklinje Philips NV.
3. What are the main segments of the Europe Wearable Medical Device Industry?
The market segments include Device Type, Application, Product Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 11.11 Million as of 2022.
5. What are some drivers contributing to market growth?
Technological Advancements and Innovation; Increasing Health Awareness; Ease of Use and Interpretation of Data.
6. What are the notable trends driving market growth?
Remote Patient Monitoring Segment is Expected to Grow Rapidly Over the Forecast Period in the Europe Wearable Medical Devices Market.
7. Are there any restraints impacting market growth?
Lack of Reimbursement Policies.
8. Can you provide examples of recent developments in the market?
IN March 2022, Infineon Technologies AG in collaboration with Sleepiz AG launched Infineon XENSIV 60 GHz radar technology, integrated into smart home and healthcare devices, that offers a great opportunity for healthcare applications as they allow to accurately measure vital signs such as heartbeat and breathing rate without touching the body.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Wearable Medical Device Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Wearable Medical Device Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Wearable Medical Device Industry?
To stay informed about further developments, trends, and reports in the Europe Wearable Medical Device Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


