Key Insights
The North America insulin biosimilars market, valued at $11.58 billion in 2025, is projected to experience robust growth, driven by several key factors. Increasing prevalence of diabetes, particularly type 1 and type 2, coupled with rising healthcare expenditure, fuels demand for cost-effective insulin therapies. Biosimilars offer a compelling alternative to brand-name insulins, providing comparable efficacy at significantly lower prices. This price advantage is a major driver, making insulin more accessible to a broader patient population and contributing to reduced healthcare costs for both individuals and insurance providers. Furthermore, the ongoing pipeline of new biosimilar insulin products entering the market is expected to intensify competition and further drive down prices, accelerating market expansion. However, challenges remain, including potential regulatory hurdles, physician and patient hesitancy towards biosimilars, and the need for robust educational campaigns to address misconceptions and foster adoption. Despite these challenges, the market's positive trajectory remains strong, supported by the inherent need for affordable insulin treatments and the continuous efforts of pharmaceutical companies to develop and market high-quality biosimilars.
The market segmentation reveals a dynamic landscape. Basal and long-acting insulin biosimilars are anticipated to hold a significant share, reflecting the high prevalence of patients requiring this type of insulin regimen. The success of individual biosimilars like Basaglar (insulin glargine) and Admelog (insulin lispro) will depend on factors such as market penetration strategies, clinical trial data, and physician prescribing habits. Combination insulin therapies and novel formulations like Soliqua/Suliqua (insulin glargine/lixisenatide) represent emerging segments with significant growth potential, catering to patients with specific needs and offering enhanced treatment benefits. Competitive pressures amongst key players including Novo Nordisk, Eli Lilly, Sanofi, and Biocon, will determine market share allocations and innovation, further shaping the market's future. The North American market, specifically the United States, is expected to remain the largest regional contributor owing to its substantial diabetic population and developed healthcare infrastructure.
North America Insulin Biosimilars Market Report: 2019-2033
This comprehensive report provides a detailed analysis of the North America insulin biosimilars market, offering invaluable insights for stakeholders across the pharmaceutical and healthcare industries. Covering the period from 2019 to 2033, with a base year of 2025, this report forecasts significant market growth and identifies key trends shaping this dynamic sector. The study meticulously examines market structure, competitive dynamics, product segments, and regulatory influences, offering actionable intelligence for informed decision-making. The report analyzes the impact of recent FDA approvals, such as Rezvoglar and Lantidra, and forecasts future market trajectory based on robust data analysis and expert projections.
North America Insulin Biosimilars Market Structure & Competitive Landscape
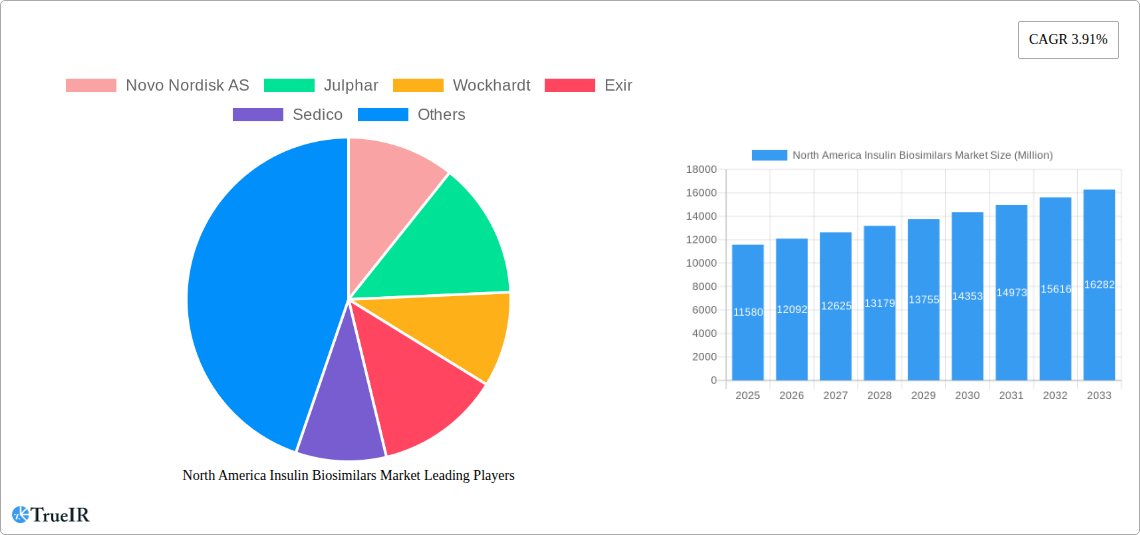
This section delves into the competitive landscape of the North American insulin biosimilars market, examining market concentration, innovation drivers, regulatory impacts, product substitutes, end-user segmentation, and M&A trends. The market is moderately concentrated, with a Herfindahl-Hirschman Index (HHI) estimated at xx in 2025. Key players like Novo Nordisk AS, Eli Lilly and Company, Sanofi S.A., and Pfizer Inc. hold significant market share, while other companies such as Biocon Limited, Julphar, Wockhardt, Exir and Sedico are actively expanding their presence.
- Market Concentration: The HHI is projected to increase slightly to xx by 2033, suggesting a potential for further consolidation through mergers and acquisitions (M&A).
- Innovation Drivers: Continuous research and development in insulin analogs and delivery systems drive innovation.
- Regulatory Impacts: FDA approvals and pricing policies significantly influence market access and competition. The approval of Rezvoglar highlights the growing acceptance of biosimilars. The impact of the recent approval of Lantidra on the biosimilar market is forecast to be xx Million by 2033.
- Product Substitutes: Traditional human insulins and other diabetes management therapies offer competition, but the cost-effectiveness of biosimilars is a crucial advantage.
- End-User Segmentation: The market is segmented by patient demographics (type 1 and type 2 diabetes) and treatment regimens.
- M&A Trends: Strategic acquisitions and partnerships are expected to continue, further shaping the market landscape. The total value of M&A deals in the North American insulin biosimilars market is estimated at xx Million during the forecast period (2025-2033).
North America Insulin Biosimilars Market Trends & Opportunities
The North America insulin biosimilars market is poised for robust growth, driven by several key factors. The market size is estimated at xx Million in 2025 and is projected to reach xx Million by 2033, exhibiting a CAGR of xx%. This growth is fueled by the increasing prevalence of diabetes, rising healthcare expenditure, and the growing acceptance of biosimilars as cost-effective alternatives to brand-name insulins. Technological advancements, particularly in delivery systems (e.g., pens, pumps), further enhance market appeal. Consumer preferences are shifting toward convenient and user-friendly insulin delivery methods, and this trend is further driving market expansion. Increased competition among biosimilar manufacturers continues to put downward pressure on pricing, making insulin more accessible to patients. The market penetration rate for insulin biosimilars is expected to increase from xx% in 2025 to xx% by 2033.
Dominant Markets & Segments in North America Insulin Biosimilars Market
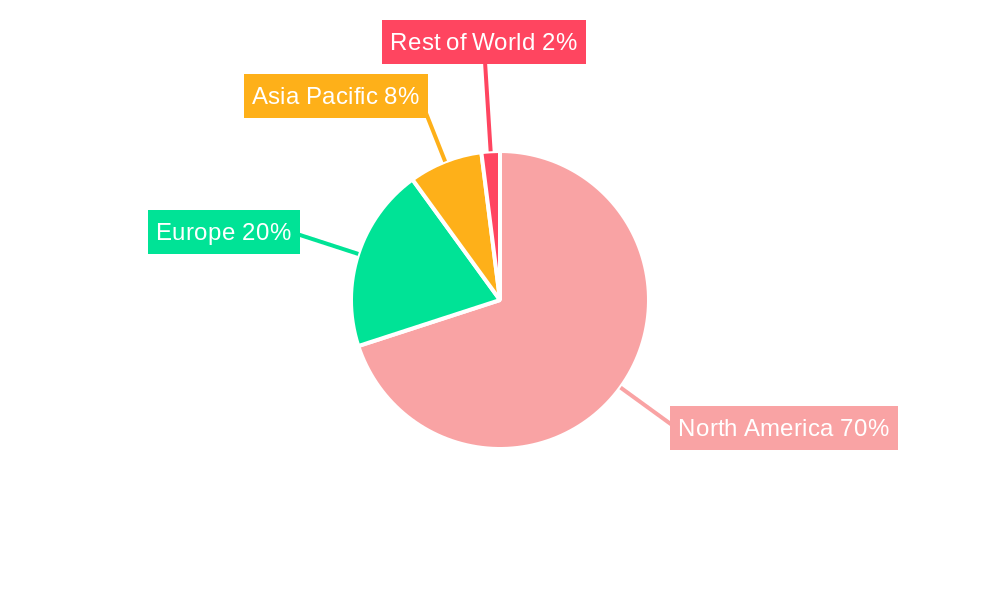
The United States dominates the North America insulin biosimilars market, accounting for the largest market share due to high diabetes prevalence and advanced healthcare infrastructure. Within the drug segments, Basal or Long-acting Insulin and Bolus or Fast-acting Insulin represent the largest market segments.
- Key Growth Drivers for the US Market:
- Robust healthcare infrastructure and high penetration of diabetes care.
- Favorable regulatory environment promoting biosimilar adoption.
- Extensive reimbursement coverage under insurance plans.
- Basal or Long-acting Insulin: This segment enjoys high demand due to its effectiveness in controlling blood glucose levels throughout the day.
- Basaglar (Insulin Glargine): Bolus or Fast-acting Insulin: This segment benefits from the established brand recognition and efficacy of insulin glargine.
- Admelog (Insulin lispro): Traditional Human Insulin: This segment appeals to price-sensitive patients and healthcare providers.
- Insuman: Combination Insulin: This segment offers benefits in terms of efficacy and convenience.
- Soliqua/Suliqua (Insulin glargine/Lixisenatide): Biosimilar Insulin: This segment holds potential for growth due to its dual mechanism of action.
North America Insulin Biosimilars Market Product Analysis
The North American insulin biosimilars market showcases a diverse range of products, including various formulations of insulin analogs (e.g., glargine, lispro, aspart) and human insulin. Technological advancements focus on improving delivery systems, such as pre-filled pens and insulin pumps, enhancing patient convenience and adherence. The competitive advantage lies in achieving biosimilarity while offering cost-effective solutions, along with enhanced delivery mechanisms and product features to improve patient compliance.
Key Drivers, Barriers & Challenges in North America Insulin Biosimilars Market
Key Drivers:
- The growing prevalence of diabetes.
- Increasing healthcare expenditure.
- Favorable regulatory environment for biosimilars (e.g., FDA approvals).
- Growing demand for cost-effective treatment options.
Challenges:
- Complex regulatory pathways for biosimilar approval can lead to delays and increase development costs.
- Supply chain disruptions can affect product availability and pricing.
- Intense competition among biosimilar manufacturers leads to price pressure.
Growth Drivers in the North America Insulin Biosimilars Market Market
The market's expansion is primarily driven by the increasing prevalence of diabetes and escalating healthcare spending. The increasing number of patients diagnosed with diabetes necessitate a greater need for insulin therapies. Favorable government regulations facilitating biosimilar entry and rising affordability are also contributing to market growth. Technological advancements, like improved delivery systems, enhance patient adherence and market demand.
Challenges Impacting North America Insulin Biosimilars Market Growth
Several factors hinder market growth. Regulatory complexities and stringent approval processes for biosimilars increase development costs and timelines. Potential supply chain disruptions can lead to shortages and price volatility. Intense competition from established players and other biosimilar manufacturers creates downward price pressures, affecting profitability.
Key Players Shaping the North America Insulin Biosimilars Market Market
- Novo Nordisk AS
- Julphar
- Wockhardt
- Exir
- Sedico
- Eli Lilly and Company
- Novo Nordisk A/S
- Other Companies
- Biocon Limited
- Pfizer Inc
- Sanofi S.A
Significant North America Insulin Biosimilars Market Industry Milestones
- November 2022: FDA approval of Rezvoglar, a second interchangeable insulin glargine biosimilar. This expands treatment options and increases competition.
- June 2023: FDA approval of Lantidra, an allogeneic pancreatic islet cellular therapy for type 1 diabetes. While not a biosimilar, this development impacts the overall diabetes treatment landscape and potentially influences patient choices and market dynamics.
Future Outlook for North America Insulin Biosimilars Market Market
The North America insulin biosimilars market is expected to experience continued growth, driven by factors such as the rising prevalence of diabetes, increasing demand for cost-effective treatments, and the ongoing development of innovative delivery systems. Strategic partnerships and collaborations among key players are likely to further drive market expansion. The market holds significant potential for growth, particularly with the continued focus on improving accessibility and affordability of insulin therapies.
North America Insulin Biosimilars Market Segmentation
-
1. Drug
- 1.1. Basal or Long-acting Insulin
- 1.2. Bolus or Fast-acting Insulin
- 1.3. Traditional Human Insulin
- 1.4. Combination Insulin
- 1.5. Biosimilar Insulin
-
2. Geography
- 2.1. United States
- 2.2. Canada
- 2.3. Rest of North America
North America Insulin Biosimilars Market Segmentation By Geography
- 1. United States
- 2. Canada
- 3. Rest of North America
North America Insulin Biosimilars Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 3.91% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; The Rise in Global Prevalence of Cases of Obesity due to Modern Sedentary Lifestyles; Rise in Awareness and Disposable Income in Developed Economies
- 3.3. Market Restrains
- 3.3.1 ; Highly Cost of Branded Products in Emerging Countries; Severe Adverse Associated with Medication Including Seizures
- 3.3.2 Suicidal Attempts and Even Death; Adoption of Traditional Yoga and Herbal Products
- 3.4. Market Trends
- 3.4.1. Basal/Long Acting Insulins Holds The Highest Market Share in Current Year
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Drug
- 5.1.1. Basal or Long-acting Insulin
- 5.1.2. Bolus or Fast-acting Insulin
- 5.1.3. Traditional Human Insulin
- 5.1.4. Combination Insulin
- 5.1.5. Biosimilar Insulin
- 5.2. Market Analysis, Insights and Forecast - by Geography
- 5.2.1. United States
- 5.2.2. Canada
- 5.2.3. Rest of North America
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. United States
- 5.3.2. Canada
- 5.3.3. Rest of North America
- 5.1. Market Analysis, Insights and Forecast - by Drug
- 6. United States North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Drug
- 6.1.1. Basal or Long-acting Insulin
- 6.1.2. Bolus or Fast-acting Insulin
- 6.1.3. Traditional Human Insulin
- 6.1.4. Combination Insulin
- 6.1.5. Biosimilar Insulin
- 6.2. Market Analysis, Insights and Forecast - by Geography
- 6.2.1. United States
- 6.2.2. Canada
- 6.2.3. Rest of North America
- 6.1. Market Analysis, Insights and Forecast - by Drug
- 7. Canada North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Drug
- 7.1.1. Basal or Long-acting Insulin
- 7.1.2. Bolus or Fast-acting Insulin
- 7.1.3. Traditional Human Insulin
- 7.1.4. Combination Insulin
- 7.1.5. Biosimilar Insulin
- 7.2. Market Analysis, Insights and Forecast - by Geography
- 7.2.1. United States
- 7.2.2. Canada
- 7.2.3. Rest of North America
- 7.1. Market Analysis, Insights and Forecast - by Drug
- 8. Rest of North America North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Drug
- 8.1.1. Basal or Long-acting Insulin
- 8.1.2. Bolus or Fast-acting Insulin
- 8.1.3. Traditional Human Insulin
- 8.1.4. Combination Insulin
- 8.1.5. Biosimilar Insulin
- 8.2. Market Analysis, Insights and Forecast - by Geography
- 8.2.1. United States
- 8.2.2. Canada
- 8.2.3. Rest of North America
- 8.1. Market Analysis, Insights and Forecast - by Drug
- 9. United States North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 10. Canada North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 11. Mexico North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 12. Rest of North America North America Insulin Biosimilars Market Analysis, Insights and Forecast, 2019-2031
- 13. Competitive Analysis
- 13.1. Market Share Analysis 2024
- 13.2. Company Profiles
- 13.2.1 Novo Nordisk AS
- 13.2.1.1. Overview
- 13.2.1.2. Products
- 13.2.1.3. SWOT Analysis
- 13.2.1.4. Recent Developments
- 13.2.1.5. Financials (Based on Availability)
- 13.2.2 Julphar
- 13.2.2.1. Overview
- 13.2.2.2. Products
- 13.2.2.3. SWOT Analysis
- 13.2.2.4. Recent Developments
- 13.2.2.5. Financials (Based on Availability)
- 13.2.3 Wockhardt
- 13.2.3.1. Overview
- 13.2.3.2. Products
- 13.2.3.3. SWOT Analysis
- 13.2.3.4. Recent Developments
- 13.2.3.5. Financials (Based on Availability)
- 13.2.4 Exir
- 13.2.4.1. Overview
- 13.2.4.2. Products
- 13.2.4.3. SWOT Analysis
- 13.2.4.4. Recent Developments
- 13.2.4.5. Financials (Based on Availability)
- 13.2.5 Sedico
- 13.2.5.1. Overview
- 13.2.5.2. Products
- 13.2.5.3. SWOT Analysis
- 13.2.5.4. Recent Developments
- 13.2.5.5. Financials (Based on Availability)
- 13.2.6 Eli Lilly and Company
- 13.2.6.1. Overview
- 13.2.6.2. Products
- 13.2.6.3. SWOT Analysis
- 13.2.6.4. Recent Developments
- 13.2.6.5. Financials (Based on Availability)
- 13.2.7 Novo Nordisk A/S
- 13.2.7.1. Overview
- 13.2.7.2. Products
- 13.2.7.3. SWOT Analysis
- 13.2.7.4. Recent Developments
- 13.2.7.5. Financials (Based on Availability)
- 13.2.8 Other Companie
- 13.2.8.1. Overview
- 13.2.8.2. Products
- 13.2.8.3. SWOT Analysis
- 13.2.8.4. Recent Developments
- 13.2.8.5. Financials (Based on Availability)
- 13.2.9 Biocon Limited
- 13.2.9.1. Overview
- 13.2.9.2. Products
- 13.2.9.3. SWOT Analysis
- 13.2.9.4. Recent Developments
- 13.2.9.5. Financials (Based on Availability)
- 13.2.10 Pfizer Inc
- 13.2.10.1. Overview
- 13.2.10.2. Products
- 13.2.10.3. SWOT Analysis
- 13.2.10.4. Recent Developments
- 13.2.10.5. Financials (Based on Availability)
- 13.2.11 Sanofi S A
- 13.2.11.1. Overview
- 13.2.11.2. Products
- 13.2.11.3. SWOT Analysis
- 13.2.11.4. Recent Developments
- 13.2.11.5. Financials (Based on Availability)
- 13.2.1 Novo Nordisk AS
List of Figures
- Figure 1: North America Insulin Biosimilars Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: North America Insulin Biosimilars Market Share (%) by Company 2024
List of Tables
- Table 1: North America Insulin Biosimilars Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: North America Insulin Biosimilars Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: North America Insulin Biosimilars Market Revenue Million Forecast, by Drug 2019 & 2032
- Table 4: North America Insulin Biosimilars Market Volume K Unit Forecast, by Drug 2019 & 2032
- Table 5: North America Insulin Biosimilars Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 6: North America Insulin Biosimilars Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 7: North America Insulin Biosimilars Market Revenue Million Forecast, by Region 2019 & 2032
- Table 8: North America Insulin Biosimilars Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 9: North America Insulin Biosimilars Market Revenue Million Forecast, by Country 2019 & 2032
- Table 10: North America Insulin Biosimilars Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 11: United States North America Insulin Biosimilars Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: United States North America Insulin Biosimilars Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 13: Canada North America Insulin Biosimilars Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Canada North America Insulin Biosimilars Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 15: Mexico North America Insulin Biosimilars Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Mexico North America Insulin Biosimilars Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 17: Rest of North America North America Insulin Biosimilars Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Rest of North America North America Insulin Biosimilars Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 19: North America Insulin Biosimilars Market Revenue Million Forecast, by Drug 2019 & 2032
- Table 20: North America Insulin Biosimilars Market Volume K Unit Forecast, by Drug 2019 & 2032
- Table 21: North America Insulin Biosimilars Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 22: North America Insulin Biosimilars Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 23: North America Insulin Biosimilars Market Revenue Million Forecast, by Country 2019 & 2032
- Table 24: North America Insulin Biosimilars Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 25: North America Insulin Biosimilars Market Revenue Million Forecast, by Drug 2019 & 2032
- Table 26: North America Insulin Biosimilars Market Volume K Unit Forecast, by Drug 2019 & 2032
- Table 27: North America Insulin Biosimilars Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 28: North America Insulin Biosimilars Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 29: North America Insulin Biosimilars Market Revenue Million Forecast, by Country 2019 & 2032
- Table 30: North America Insulin Biosimilars Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 31: North America Insulin Biosimilars Market Revenue Million Forecast, by Drug 2019 & 2032
- Table 32: North America Insulin Biosimilars Market Volume K Unit Forecast, by Drug 2019 & 2032
- Table 33: North America Insulin Biosimilars Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 34: North America Insulin Biosimilars Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 35: North America Insulin Biosimilars Market Revenue Million Forecast, by Country 2019 & 2032
- Table 36: North America Insulin Biosimilars Market Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the North America Insulin Biosimilars Market?
The projected CAGR is approximately 3.91%.
2. Which companies are prominent players in the North America Insulin Biosimilars Market?
Key companies in the market include Novo Nordisk AS, Julphar, Wockhardt, Exir, Sedico, Eli Lilly and Company, Novo Nordisk A/S, Other Companie, Biocon Limited, Pfizer Inc, Sanofi S A.
3. What are the main segments of the North America Insulin Biosimilars Market?
The market segments include Drug, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 11.58 Million as of 2022.
5. What are some drivers contributing to market growth?
; The Rise in Global Prevalence of Cases of Obesity due to Modern Sedentary Lifestyles; Rise in Awareness and Disposable Income in Developed Economies.
6. What are the notable trends driving market growth?
Basal/Long Acting Insulins Holds The Highest Market Share in Current Year.
7. Are there any restraints impacting market growth?
; Highly Cost of Branded Products in Emerging Countries; Severe Adverse Associated with Medication Including Seizures. Suicidal Attempts and Even Death; Adoption of Traditional Yoga and Herbal Products.
8. Can you provide examples of recent developments in the market?
June 2023: The initial allogeneic (donor) pancreatic islet cellular therapy, Lantidra, has been sanctioned by the U.S. Food and Drug Administration. This treatment is derived from pancreatic cells of deceased donors and is intended for individuals with type 1 diabetes. Lantidra is specifically authorized for adults who struggle to achieve target glycated hemoglobin levels due to frequent severe hypoglycemia episodes, despite undergoing intensive diabetes management and education.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "North America Insulin Biosimilars Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the North America Insulin Biosimilars Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the North America Insulin Biosimilars Market?
To stay informed about further developments, trends, and reports in the North America Insulin Biosimilars Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


