Key Insights
The Argentina Cardiovascular Devices Market is projected for substantial growth, reaching an estimated market size of 4.48 billion by 2024. This expansion is driven by a projected Compound Annual Growth Rate (CAGR) of 5.53%. Key growth factors include the rising incidence of cardiovascular diseases (CVDs) in Argentina, increased patient awareness of preventive healthcare, and advancements in medical technology for diagnosis and treatment. The aging Argentine population also contributes to higher demand for cardiovascular interventions and monitoring devices. Emerging trends such as the adoption of remote cardiac monitoring systems for continuous patient oversight and early anomaly detection, alongside the increasing use of minimally invasive surgical techniques requiring advanced devices like catheters, grafts, and heart valves, are shaping market dynamics. Furthermore, a growing focus on interventional cardiology procedures, supported by innovative stents and cardiac assist devices, is a pivotal factor driving market penetration.
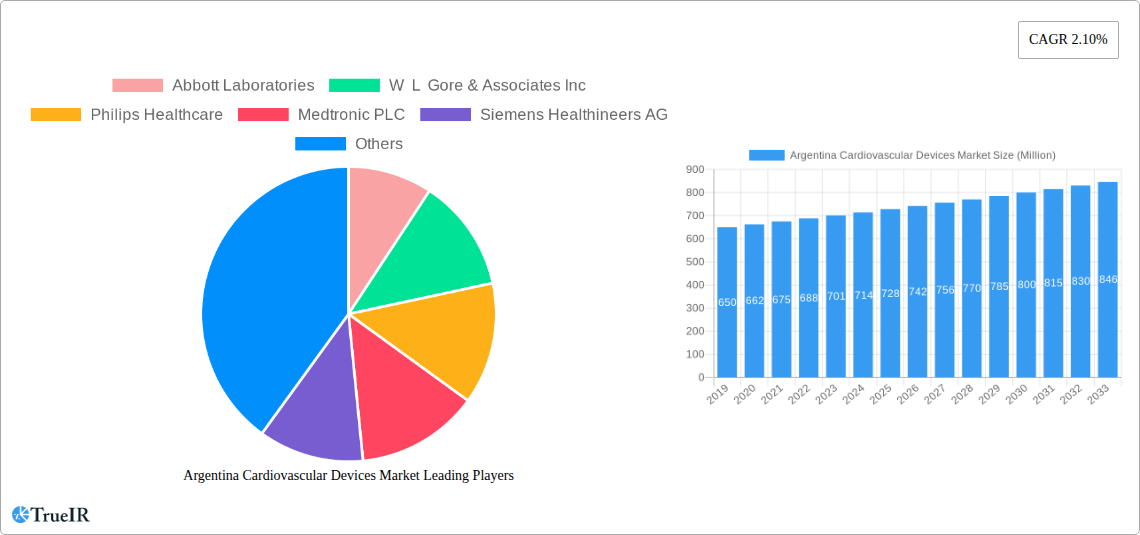
Argentina Cardiovascular Devices Market Market Size (In Billion)

Despite the positive growth forecast, the market faces challenges. Economic volatility and potential healthcare budget constraints in Argentina may affect the procurement of advanced medical devices. The high cost of sophisticated cardiovascular technologies and potential reimbursement policy complexities can also impede widespread adoption. Market segmentation indicates robust demand for both diagnostic and monitoring devices, with Electrocardiogram (ECG) and remote cardiac monitoring systems leading. Therapeutic and surgical devices, including cardiac rhythm management systems and implants, are also experiencing significant growth. Leading companies like Medtronic PLC, Abbott Laboratories, and Philips Healthcare are actively contributing with innovative product portfolios, enhancing the accessibility of advanced cardiovascular care in Argentina.
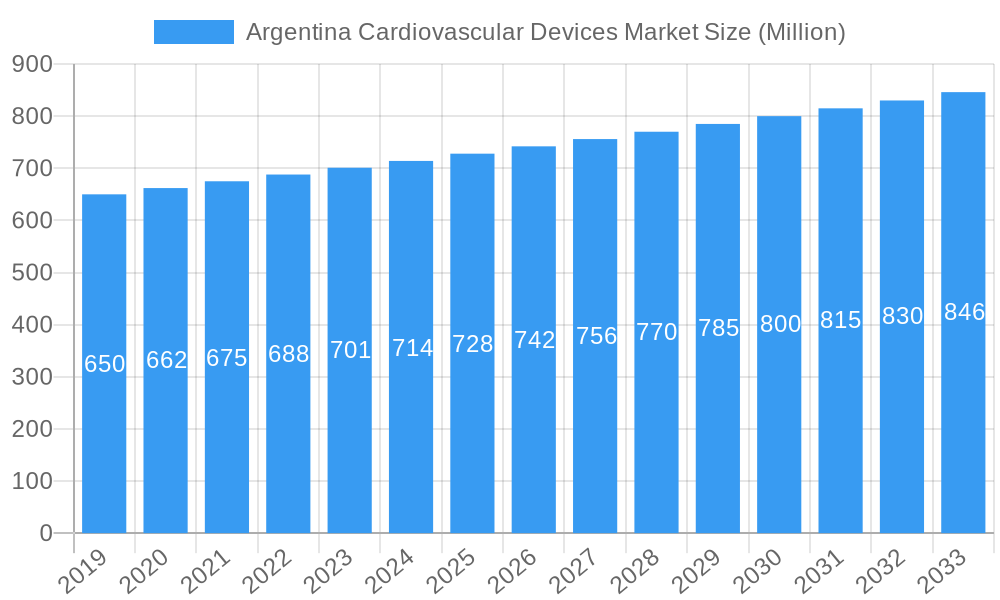
Argentina Cardiovascular Devices Market Company Market Share

This comprehensive report offers an in-depth analysis of the Argentina Cardiovascular Devices Market, providing a detailed forecast from 2024 to 2033, with 2024 as the base year. We explore critical market dynamics, including cardiac monitoring devices, cardiac rhythm management devices, stents, heart valves, and cardiovascular implants. This study delivers actionable insights for stakeholders aiming to leverage the significant opportunities within Argentina's expanding healthcare sector, fueled by the increasing prevalence of cardiovascular diseases and rising healthcare expenditure.
Argentina Cardiovascular Devices Market Market Structure & Competitive Landscape
The Argentina Cardiovascular Devices Market exhibits a moderately concentrated structure, with key global players vying for market share alongside emerging local manufacturers. Innovation remains a pivotal driver, fueled by advancements in minimally invasive technologies and remote patient monitoring solutions. Regulatory impacts, particularly from the Argentine National Administration of Drugs, Foods, and Medical Devices (ANMAT), play a significant role in market entry and product adoption. While direct product substitutes are limited in scope, the increasing adoption of lifestyle modifications and preventative healthcare can be considered an indirect substitute. End-user segmentation spans hospitals, clinics, diagnostic centers, and ambulatory surgical centers, each with distinct purchasing patterns and device requirements. Mergers and acquisitions (M&A) trends, though currently moderate, are expected to accelerate as larger entities seek to expand their product portfolios and geographic reach within Argentina. The market's concentration ratio is estimated to be around 45% for the top five players. M&A activities in the historical period (2019-2024) involved approximately 2 significant transactions focused on technology acquisition.
Argentina Cardiovascular Devices Market Market Trends & Opportunities
The Argentina Cardiovascular Devices Market is poised for substantial growth, projecting a Compound Annual Growth Rate (CAGR) of approximately 7.8% during the forecast period of 2025–2033. This upward trajectory is intrinsically linked to the increasing prevalence of cardiovascular diseases (CVDs) within the Argentine population, estimated to affect over 30% of adults. Technological shifts are profoundly shaping the market, with a strong emphasis on the development and adoption of advanced diagnostic and monitoring devices. This includes a growing demand for sophisticated electrocardiogram (ECG) devices, particularly portable and wearable solutions, alongside a surge in the adoption of remote cardiac monitoring systems. These technologies empower healthcare providers to continuously track patient cardiac health, enabling early detection and intervention, thereby reducing hospitalization rates.
Furthermore, the therapeutic segment is witnessing significant innovation, particularly in cardiac rhythm management devices and catheters. The increasing number of interventional cardiology procedures, driven by a growing preference for less invasive treatments, is a key growth catalyst. The market penetration of advanced stents, including drug-eluting stents (DES), is steadily increasing, offering improved patient outcomes and reduced restenosis rates. Similarly, the demand for innovative heart valves, particularly transcatheter aortic valve implantation (TAVI) devices, is on the rise, catering to an aging population with a higher incidence of valvular heart disease.
Consumer preferences are evolving, with a greater awareness of cardiovascular health and a proactive approach towards managing existing conditions. Patients are increasingly seeking solutions that offer convenience, improved quality of life, and better long-term prognoses. This shift is driving the demand for implantable devices with enhanced functionality and longevity. Competitive dynamics are characterized by intense R&D efforts aimed at developing next-generation technologies. Key players are focusing on expanding their product pipelines, strategic partnerships, and robust distribution networks to capture a larger market share. The market size is estimated to reach approximately $650 Million by 2033.
Dominant Markets & Segments in Argentina Cardiovascular Devices Market
The Diagnostic and Monitoring Devices segment is currently the dominant force within the Argentina Cardiovascular Devices Market, driven by an increasing focus on early detection and preventative healthcare. Within this segment, Electrocardiogram (ECG) devices hold a significant share, fueled by the need for accurate and accessible cardiac diagnostics in various healthcare settings. The rising prevalence of cardiovascular conditions necessitates continuous monitoring, propelling the growth of remote cardiac monitoring solutions. These systems, encompassing wearable sensors and telehealth platforms, are gaining traction due to their ability to provide real-time data and facilitate proactive patient management, especially for chronic conditions.
The Therapeutic and Surgical Devices segment is also a critical contributor and is expected to witness robust growth. Cardiac rhythm management devices, including pacemakers and implantable cardioverter-defibrillators (ICDs), are in high demand due to the aging population and increasing incidence of arrhythmias. Catheters, particularly those used in minimally invasive procedures like angioplasty and stenting, are experiencing significant market penetration. Stents, especially advanced drug-eluting stents, are crucial for treating coronary artery disease, a prevalent condition in Argentina. The demand for heart valves, particularly for TAVI procedures, is also on an upward trend, addressing the growing burden of valvular heart disease.
Key Growth Drivers for Diagnostic and Monitoring Devices:
- Increasing government initiatives promoting early disease detection.
- Growing adoption of wearable technology for personal health monitoring.
- Expansion of telehealth infrastructure and services.
- Technological advancements leading to more accurate and user-friendly devices.
Key Growth Drivers for Therapeutic and Surgical Devices:
- Rising incidence of cardiovascular diseases and associated procedures.
- Preference for minimally invasive surgical techniques.
- Development of advanced biomaterials for implants and grafts.
- Increasing healthcare expenditure and insurance coverage for cardiac treatments.
The market dominance of these segments is underpinned by strong policy support for cardiovascular health initiatives and a growing network of specialized cardiac care centers across Argentina. The estimated market size for Diagnostic and Monitoring Devices is $280 Million in 2025, while Therapeutic and Surgical Devices are projected at $370 Million in the same year.
Argentina Cardiovascular Devices Market Product Analysis
The Argentina Cardiovascular Devices Market is characterized by a stream of innovative products designed to enhance diagnostic accuracy, improve treatment efficacy, and elevate patient quality of life. Technological advancements are evident in the miniaturization of devices, the integration of artificial intelligence for data analysis, and the development of novel biomaterials for implants. For instance, next-generation electrocardiogram (ECG) devices offer enhanced signal processing and remote data transmission capabilities, while cardiac assist devices are becoming more sophisticated, offering improved hemodynamic support with reduced invasiveness. The competitive advantage lies in devices that demonstrate superior performance, patient safety, and cost-effectiveness, catering to the evolving needs of both healthcare providers and patients.
Key Drivers, Barriers & Challenges in Argentina Cardiovascular Devices Market
The Argentina Cardiovascular Devices Market is propelled by several key drivers including the rising incidence of cardiovascular diseases, increasing government focus on public health, and continuous technological innovation in diagnostic and therapeutic devices. Economic growth and improved healthcare infrastructure also contribute significantly.
However, the market faces considerable barriers and challenges. Regulatory complexities and lengthy approval processes can hinder the timely introduction of new products. Supply chain disruptions, particularly for imported components and finished goods, pose a significant risk, potentially leading to stockouts and increased costs. Intense competition among domestic and international players, coupled with price sensitivity from healthcare providers, creates pressure on profit margins. Furthermore, the accessibility and affordability of advanced cardiovascular devices for a wider population remain a challenge.
Growth Drivers in the Argentina Cardiovascular Devices Market Market
Several factors are fueling the growth of the Argentina Cardiovascular Devices Market. Technologically, the advent of sophisticated remote cardiac monitoring systems and advanced stents is driving demand. Economically, increasing disposable incomes and a growing healthcare expenditure budget within Argentina are making advanced treatments more accessible. Regulatory support for medical device innovation and the establishment of specialized cardiac care centers are also significant growth enablers. The expanding geriatric population, more susceptible to cardiovascular ailments, further bolsters the market.
Challenges Impacting Argentina Cardiovascular Devices Market Growth
Challenges impacting the Argentina Cardiovascular Devices Market include stringent and sometimes unpredictable regulatory landscapes, leading to extended product launch timelines and increased compliance costs, estimated at around 15% of R&D expenses. Supply chain vulnerabilities, exacerbated by global logistics issues, can cause delays and price fluctuations, impacting the availability of critical components and finished devices. Competitive pressures from both established global brands and emerging local manufacturers can lead to price erosion, affecting profitability. Furthermore, ensuring equitable access to high-cost advanced cardiovascular technologies across diverse socioeconomic groups remains a persistent challenge.
Key Players Shaping the Argentina Cardiovascular Devices Market Market
- Abbott Laboratories
- W L Gore & Associates Inc
- Philips Healthcare
- Medtronic PLC
- Siemens Healthineers AG
- Stryker
- Canon Medical Systems Corporation
- Biosud Argentina
- Welch Allyn Inc
- Nihon Kohden Corporation
Significant Argentina Cardiovascular Devices Market Industry Milestones
- March 2022: MicroPort CardioFlow Medtech Corporation received marketing approval from the Argentine National Administration of Drugs, Foods, and Medical Devices (ANMAT) for their Alwide Plus Balloon Catheter (Alwide Plus). This approval is expected to enhance treatment options for aortic stenosis.
- January 2022: The Argentine National Administration of Drugs, Foods, and Medical Devices (ANMAT) approved the VitaFlow Transcatheter Aortic Valve and Delivery System by MicroPort CardioFlow Medtech Corporation. This marked a significant step in expanding access to minimally invasive valve replacement procedures in Argentina.
Future Outlook for Argentina Cardiovascular Devices Market Market
The future outlook for the Argentina Cardiovascular Devices Market is exceptionally promising, driven by sustained demographic trends and a commitment to enhancing cardiovascular healthcare. The increasing adoption of minimally invasive technologies, coupled with the ongoing evolution of diagnostic and monitoring devices, will continue to fuel market expansion. Strategic collaborations between global manufacturers and local distributors are anticipated to broaden market reach and improve access to advanced medical technologies. Furthermore, growing investments in healthcare infrastructure and a continued emphasis on preventative medicine will create a fertile ground for sustained growth and innovation in the years to come, with a projected market size reaching over $800 Million by 2033.
Argentina Cardiovascular Devices Market Segmentation
-
1. Device Type
-
1.1. Diagnostic and Monitoring Devices
- 1.1.1. Electrocardiogram (ECG)
- 1.1.2. Remote Cardiac Monitoring
- 1.1.3. Other Diagnostic and Monitoring Devices
-
1.2. Therapeutic and Surgical Devices
- 1.2.1. Cardiac Assist Devices
- 1.2.2. Cardiac Rhythm Management Devices
- 1.2.3. Catheter
- 1.2.4. Grafts
- 1.2.5. Heart Valves
- 1.2.6. Stents
- 1.2.7. Other Therapeutic and Surgical Devices
-
1.1. Diagnostic and Monitoring Devices
Argentina Cardiovascular Devices Market Segmentation By Geography
- 1. Argentina
Argentina Cardiovascular Devices Market Regional Market Share

Geographic Coverage of Argentina Cardiovascular Devices Market
Argentina Cardiovascular Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.53% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Burden of Cardiovascular Diseases; Increased Preference of Minimally Invasive Procedures
- 3.3. Market Restrains
- 3.3.1. Stringent Regulatory Policies; High Cost of Instruments and Procedures
- 3.4. Market Trends
- 3.4.1. Electrocardiogram (ECG) Sub-segment is Expected to Witness Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Argentina Cardiovascular Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Diagnostic and Monitoring Devices
- 5.1.1.1. Electrocardiogram (ECG)
- 5.1.1.2. Remote Cardiac Monitoring
- 5.1.1.3. Other Diagnostic and Monitoring Devices
- 5.1.2. Therapeutic and Surgical Devices
- 5.1.2.1. Cardiac Assist Devices
- 5.1.2.2. Cardiac Rhythm Management Devices
- 5.1.2.3. Catheter
- 5.1.2.4. Grafts
- 5.1.2.5. Heart Valves
- 5.1.2.6. Stents
- 5.1.2.7. Other Therapeutic and Surgical Devices
- 5.1.1. Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Argentina
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Abbott Laboratories
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 W L Gore & Associates Inc
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Philips Healthcare
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Medtronic PLC
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Siemens Healthineers AG
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Stryker
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Canon Medical Systems Corporation
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Biosud Argentina
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Welch Allyn Inc
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Nihon Kohden Corporation
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.1 Abbott Laboratories
List of Figures
- Figure 1: Argentina Cardiovascular Devices Market Revenue Breakdown (billion, %) by Product 2025 & 2033
- Figure 2: Argentina Cardiovascular Devices Market Share (%) by Company 2025
List of Tables
- Table 1: Argentina Cardiovascular Devices Market Revenue billion Forecast, by Device Type 2020 & 2033
- Table 2: Argentina Cardiovascular Devices Market Volume K Unit Forecast, by Device Type 2020 & 2033
- Table 3: Argentina Cardiovascular Devices Market Revenue billion Forecast, by Region 2020 & 2033
- Table 4: Argentina Cardiovascular Devices Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 5: Argentina Cardiovascular Devices Market Revenue billion Forecast, by Device Type 2020 & 2033
- Table 6: Argentina Cardiovascular Devices Market Volume K Unit Forecast, by Device Type 2020 & 2033
- Table 7: Argentina Cardiovascular Devices Market Revenue billion Forecast, by Country 2020 & 2033
- Table 8: Argentina Cardiovascular Devices Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Argentina Cardiovascular Devices Market?
The projected CAGR is approximately 5.53%.
2. Which companies are prominent players in the Argentina Cardiovascular Devices Market?
Key companies in the market include Abbott Laboratories, W L Gore & Associates Inc, Philips Healthcare, Medtronic PLC, Siemens Healthineers AG, Stryker, Canon Medical Systems Corporation, Biosud Argentina, Welch Allyn Inc, Nihon Kohden Corporation.
3. What are the main segments of the Argentina Cardiovascular Devices Market?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 4.48 billion as of 2022.
5. What are some drivers contributing to market growth?
Increasing Burden of Cardiovascular Diseases; Increased Preference of Minimally Invasive Procedures.
6. What are the notable trends driving market growth?
Electrocardiogram (ECG) Sub-segment is Expected to Witness Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
Stringent Regulatory Policies; High Cost of Instruments and Procedures.
8. Can you provide examples of recent developments in the market?
In March 2022, MicroPort CardioFlow Medtech Corporation indicated that their Alwide Plus Balloon Catheter (Alwide Plus) had received marketing approval from the Argentine National Administration of Drugs, Foods, and Medical Devices (ANMAT).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Argentina Cardiovascular Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Argentina Cardiovascular Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Argentina Cardiovascular Devices Market?
To stay informed about further developments, trends, and reports in the Argentina Cardiovascular Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


