Key Insights
The global Chronic Myelogenous Leukemia (CML) Treatment market is projected for substantial growth, expected to reach USD 9264.8 million by 2025, with a Compound Annual Growth Rate (CAGR) of 5.6%. This expansion is driven by advancements in targeted therapies, offering improved efficacy and reduced side effects compared to conventional chemotherapy. Increasing CML incidence, heightened disease awareness, and enhanced diagnostic tools further fuel market growth. The robust pipeline of innovative biologic and combination therapies also promises to elevate patient outcomes and market expansion. The market size is presented in millions of USD.
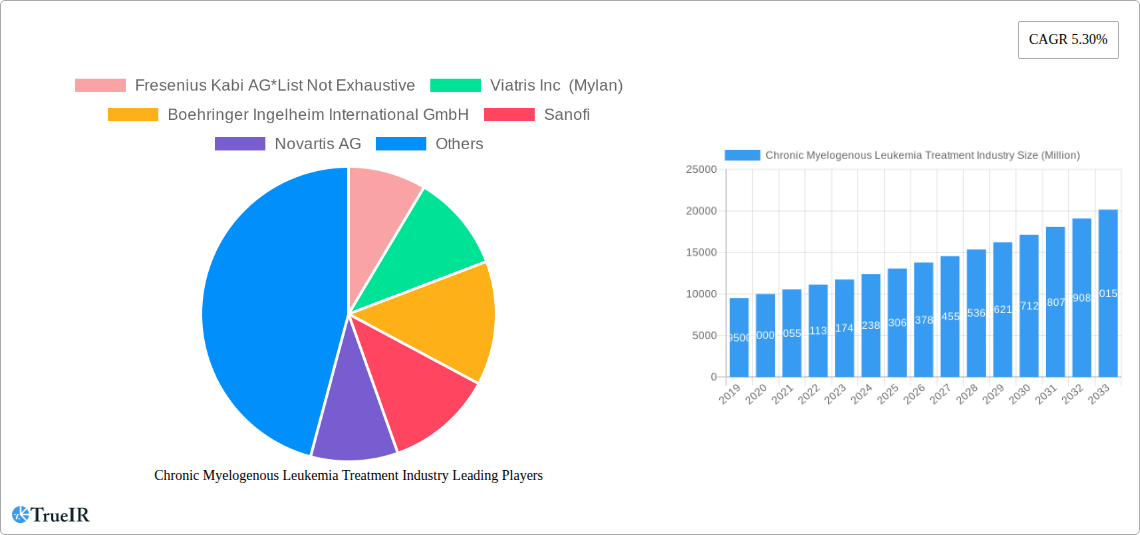
Chronic Myelogenous Leukemia Treatment Industry Market Size (In Billion)

Key factors influencing the CML Treatment market include the high cost of novel targeted therapies, posing an accessibility challenge, particularly in emerging economies. Stringent regulatory approval pathways for new drug candidates also present hurdles. Nevertheless, efforts to enhance affordability and accessibility through patient support programs and biosimilar development are underway. Leading market participants are prioritizing R&D for next-generation treatments, focusing on overcoming resistance and achieving sustained disease control. North America and Europe are anticipated to lead market share due to advanced healthcare infrastructure and high adoption rates of novel therapies. The Asia Pacific region is poised for the fastest growth, supported by increasing healthcare investments and a growing patient demographic.
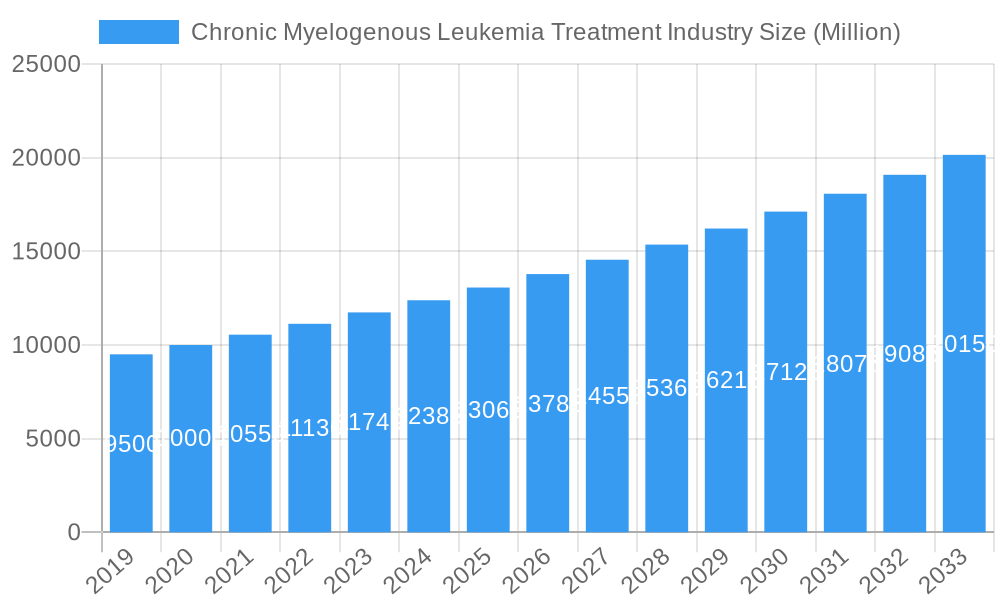
Chronic Myelogenous Leukemia Treatment Industry Company Market Share

Chronic Myelogenous Leukemia Treatment Industry Market Research Report: Driving Innovation and Global Growth (2019-2033)
This comprehensive report offers an in-depth analysis of the global Chronic Myelogenous Leukemia (CML) Treatment Industry, providing critical insights for stakeholders. Covering the historical period of 2019-2024 and projecting growth through 2033 with a base year of 2025, this research delves into market structure, trends, opportunities, key players, and future outlook. Leveraging high-volume keywords such as "CML treatment," "leukemia therapy," "targeted therapy," "chemotherapy for CML," and "biologic therapy for leukemia," this report is optimized for search engine visibility and designed to engage industry professionals, researchers, and investors.
Chronic Myelogenous Leukemia Treatment Industry Market Structure & Competitive Landscape
The Chronic Myelogenous Leukemia (CML) Treatment Industry is characterized by a moderately concentrated market, driven by significant research and development investment and stringent regulatory approvals. Innovation plays a pivotal role, with a continuous influx of novel therapies, particularly in the targeted therapy segment. Regulatory impacts from bodies like the FDA and EMA significantly influence market entry and product lifecycle management. Product substitutes, while limited in advanced treatment lines, can emerge from improvements in existing drug classes or the development of entirely new therapeutic modalities. End-user segmentation primarily includes academic medical centers, hospitals, and specialized cancer treatment clinics. Merger and acquisition (M&A) trends are observed as major pharmaceutical companies seek to expand their oncology portfolios and acquire promising pipeline assets. For instance, in recent years, M&A volumes have been approximately $3,000 Million annually, consolidating market share. Key competitive advantages lie in patent protection, clinical trial success, and the ability to secure favorable reimbursement policies.
Chronic Myelogenous Leukemia Treatment Industry Market Trends & Opportunities
The global Chronic Myelogenous Leukemia (CML) Treatment Industry is poised for substantial growth, projected to reach a market size of over $15,000 Million by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of approximately 7.5% during the forecast period of 2025-2033. This expansion is fueled by increasing CML incidence rates, improved diagnostic capabilities leading to earlier detection, and advancements in therapeutic efficacy. Technological shifts are prominently centered around the development of next-generation tyrosine kinase inhibitors (TKIs) that demonstrate enhanced potency, reduced side effects, and the ability to overcome drug resistance. Consumer preferences are increasingly leaning towards personalized medicine approaches, demanding treatments tailored to individual genetic profiles and disease subtypes. Competitive dynamics are intensifying as established players and emerging biopharmaceutical companies vie for market dominance through pipeline innovation and strategic partnerships. The market penetration of advanced targeted therapies continues to rise, displacing traditional chemotherapy options in many treatment protocols. Opportunities abound in developing cost-effective treatments, expanding access in emerging markets, and exploring novel therapeutic combinations to address refractory CML cases. Furthermore, the growing emphasis on patient quality of life and survivorship is driving demand for therapies with favorable long-term safety profiles. The market is also witnessing a surge in investments in personalized CML management, leveraging companion diagnostics to guide treatment decisions.
Dominant Markets & Segments in Chronic Myelogenous Leukemia Treatment Industry
The Targeted Therapy segment is the undisputed leader within the Chronic Myelogenous Leukemia (CML) Treatment Industry, commanding an estimated 70% of the market share in the base year of 2025. This dominance is attributed to the revolutionary impact of TKIs, which have transformed CML from a fatal illness into a manageable chronic condition.
- North America stands as the dominant regional market, accounting for approximately 45% of the global CML treatment revenue in 2025. This leadership is driven by high healthcare spending, advanced medical infrastructure, robust reimbursement policies, and a high prevalence of CML diagnoses. The United States, in particular, represents a significant portion of this regional dominance due to its extensive network of specialized cancer centers and rapid adoption of innovative therapies.
Key growth drivers for the targeted therapy segment and dominant regions include:
- Technological Advancements: Continuous development of more potent and selective TKIs with improved resistance profiles, such as third-generation TKIs, is a primary growth catalyst.
- Early Diagnosis and Screening: Enhanced diagnostic tools and increased awareness facilitate earlier identification of CML, leading to prompt initiation of targeted therapies.
- Favorable Reimbursement Policies: Government and private insurance coverage for expensive targeted therapies in developed nations supports market expansion.
- Research and Development Investment: Significant R&D spending by major pharmaceutical companies fuels the pipeline of novel CML treatments.
- Patient Advocacy and Awareness: Growing patient advocacy groups contribute to increased awareness and demand for effective CML treatments.
- Clinical Guidelines: Evolving treatment guidelines that recommend targeted therapies as first-line treatment solidify their market position.
While Chemotherapy and Biologic Therapy segments still hold a presence, their market share is comparatively smaller, estimated at 15% and 10% respectively in 2025, primarily for specific patient populations or as adjunct treatments. The "Other Treatment Types" segment, encompassing supportive care and emerging therapies, is projected to grow at a faster rate, albeit from a smaller base. The ability to overcome TKI resistance remains a significant area of unmet need, creating opportunities for innovative approaches within these segments.
Chronic Myelogenous Leukemia Treatment Industry Product Analysis
Product innovation in the Chronic Myelogenous Leukemia (CML) Treatment Industry is primarily centered on the development of novel tyrosine kinase inhibitors (TKIs) with improved efficacy and safety profiles. Next-generation TKIs are designed to overcome resistance mechanisms, such as BCR-ABL mutations, that can limit the effectiveness of earlier-generation drugs. These advancements offer patients more durable responses and a better quality of life. Competitive advantages are derived from demonstrating superior clinical outcomes, favorable pharmacokinetic properties, and the ability to target specific patient populations. The market fit for these products is strong, given the increasing demand for more personalized and effective CML management strategies.
Key Drivers, Barriers & Challenges in Chronic Myelogenous Leukemia Treatment Industry
Key Drivers: The Chronic Myelogenous Leukemia Treatment Industry is propelled by significant drivers, including the increasing global incidence of CML, advancements in diagnostic technologies leading to earlier detection, and the continuous development of highly effective targeted therapies like tyrosine kinase inhibitors (TKIs). Growing research and development investments by pharmaceutical giants, coupled with favorable reimbursement policies in developed nations, further accelerate market growth. The increasing emphasis on personalized medicine and patient-centric treatment approaches also acts as a significant catalyst.
Barriers & Challenges: Despite the positive outlook, the industry faces several challenges. The high cost of advanced CML therapies poses a significant barrier to access, particularly in low- and middle-income countries. Stringent regulatory hurdles and lengthy approval processes for new drug applications can delay market entry. Supply chain complexities and potential drug shortages can also impact availability. Furthermore, the emergence of drug resistance mechanisms and the need for continuous treatment present ongoing challenges for long-term patient management. Intense competition among established and emerging players also necessitates significant investment in marketing and clinical studies.
Growth Drivers in the Chronic Myelogenous Leukemia Treatment Industry Market
The growth of the Chronic Myelogenous Leukemia Treatment Industry is primarily driven by several key factors. Technological advancements in targeted therapies, particularly the development of novel tyrosine kinase inhibitors (TKIs) with enhanced potency and resistance-breaking capabilities, are paramount. Economic factors, such as increasing healthcare expenditure globally and favorable reimbursement policies in major markets like North America and Europe, support market expansion. Regulatory drivers include supportive government initiatives for oncology drug development and streamlined approval pathways for innovative treatments. Specific examples include the continuous pipeline of next-generation TKIs aiming to address unmet needs in TKI-resistant CML.
Challenges Impacting Chronic Myelogenous Leukemia Treatment Industry Growth
Several challenges impact the growth of the Chronic Myelogenous Leukemia Treatment Industry. Regulatory complexities, including stringent clinical trial requirements and post-market surveillance, can delay product launches. Supply chain issues, from raw material sourcing to final product distribution, can create vulnerabilities. Competitive pressures are high, with multiple companies vying for market share, necessitating significant investment in research and development, marketing, and sales. The high cost of innovative CML treatments is a significant barrier to accessibility, particularly in emerging economies, and can lead to pricing pressures from payers. Additionally, the development of drug resistance mechanisms by CML cells requires continuous innovation to maintain treatment efficacy.
Key Players Shaping the Chronic Myelogenous Leukemia Treatment Industry Market
- Fresenius Kabi AG
- Viatris Inc (Mylan)
- Boehringer Ingelheim International GmbH
- Sanofi
- Novartis AG
- Cipla Inc (Cipla USA Inc)
- Bristol-Myers Squibb Co
- Merck & Co Inc
- F Hoffmann-La Roche Ltd
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd
- Accord Healthcare Inc
- Amneal Pharmaceuticals LLC
- Pfizer Inc
Significant Chronic Myelogenous Leukemia Treatment Industry Industry Milestones
- December 2021: Ascentage Pharma launched its innovative drug Olverembatinib in China, marking a significant advancement in treating adult patients with chronic or accelerated phase chronic myeloid leukemia (CML). Olverembatinib, manufactured by Guangzhou HealthQuest Pharma Co Ltd Inc, is the first of its kind launched in China.
- July 2022: The Center for Drug Evaluation (CDE) of China of the National Medical Products Administration (NMPA) accepted and granted Priority Review designation to a New Drug Application (NDA) submitted by Innovent Biologics, Inc. and Ascentage Pharma for olverembatinib. This designation supports the full approval of the drug for patients with chronic-phase CML who are resistant or intolerant to first- and second-generation TKIs, signifying a crucial step towards broader patient access.
Future Outlook for Chronic Myelogenous Leukemia Treatment Industry Market
The future outlook for the Chronic Myelogenous Leukemia Treatment Industry is exceptionally promising, driven by ongoing advancements in targeted therapies and a growing understanding of CML biology. Strategic opportunities lie in the development of treatments for TKI-resistant CML, personalized medicine approaches leveraging genomics, and the expansion of access to effective therapies in underserved markets. The market potential is significant, with continued innovation expected to drive substantial revenue growth and improve patient outcomes globally. The increasing focus on combination therapies and the exploration of novel therapeutic modalities will further shape the treatment landscape, solidifying CML as a manageable chronic disease for the vast majority of patients.
Chronic Myelogenous Leukemia Treatment Industry Segmentation
-
1. Treatment Type
- 1.1. Targeted therapy
- 1.2. Chemotherapy
- 1.3. Biologic therapy
- 1.4. Other Treatment Types
Chronic Myelogenous Leukemia Treatment Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
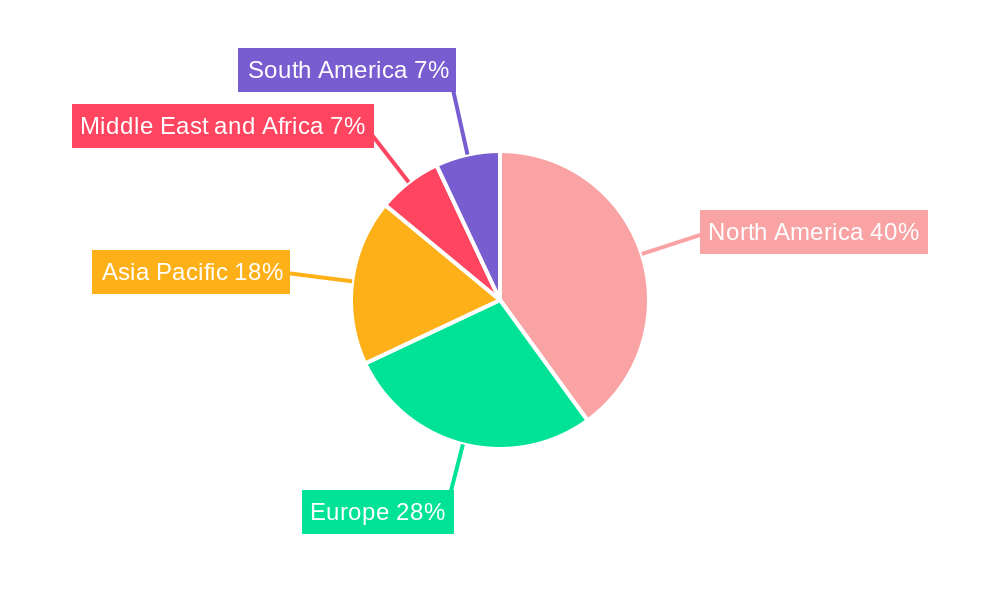
Chronic Myelogenous Leukemia Treatment Industry Regional Market Share

Geographic Coverage of Chronic Myelogenous Leukemia Treatment Industry
Chronic Myelogenous Leukemia Treatment Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.6% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. High Incidence and Prevalence of Chronic Myeloid Leukemia; Advancement in Drug Development; Increasing Investments in Research and Development
- 3.3. Market Restrains
- 3.3.1. Side effects Associated with Chemotherapy; Stringent Regulations on Drugs
- 3.4. Market Trends
- 3.4.1. The Chemotherapy Segment is Expected to Witness Significant Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Treatment Type
- 5.1.1. Targeted therapy
- 5.1.2. Chemotherapy
- 5.1.3. Biologic therapy
- 5.1.4. Other Treatment Types
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.2.2. Europe
- 5.2.3. Asia Pacific
- 5.2.4. Middle East and Africa
- 5.2.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Treatment Type
- 6. North America Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Treatment Type
- 6.1.1. Targeted therapy
- 6.1.2. Chemotherapy
- 6.1.3. Biologic therapy
- 6.1.4. Other Treatment Types
- 6.1. Market Analysis, Insights and Forecast - by Treatment Type
- 7. Europe Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Treatment Type
- 7.1.1. Targeted therapy
- 7.1.2. Chemotherapy
- 7.1.3. Biologic therapy
- 7.1.4. Other Treatment Types
- 7.1. Market Analysis, Insights and Forecast - by Treatment Type
- 8. Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Treatment Type
- 8.1.1. Targeted therapy
- 8.1.2. Chemotherapy
- 8.1.3. Biologic therapy
- 8.1.4. Other Treatment Types
- 8.1. Market Analysis, Insights and Forecast - by Treatment Type
- 9. Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Treatment Type
- 9.1.1. Targeted therapy
- 9.1.2. Chemotherapy
- 9.1.3. Biologic therapy
- 9.1.4. Other Treatment Types
- 9.1. Market Analysis, Insights and Forecast - by Treatment Type
- 10. South America Chronic Myelogenous Leukemia Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Treatment Type
- 10.1.1. Targeted therapy
- 10.1.2. Chemotherapy
- 10.1.3. Biologic therapy
- 10.1.4. Other Treatment Types
- 10.1. Market Analysis, Insights and Forecast - by Treatment Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Fresenius Kabi AG*List Not Exhaustive
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Viatris Inc (Mylan)
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Boehringer Ingelheim International GmbH
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Sanofi
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Novartis AG
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Cipla Inc (Cipla USA Inc )
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Bristol-Myers Squibb Co
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Merck & Co Inc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 F Hoffmann-La Roche Ltd
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Takeda Pharmaceutical Company Limited
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Teva Pharmaceutical Industries Ltd
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Accord Healthcare Inc
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Amneal Pharmaceuticals LLC
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Pfizer Inc
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.1 Fresenius Kabi AG*List Not Exhaustive
List of Figures
- Figure 1: Global Chronic Myelogenous Leukemia Treatment Industry Revenue Breakdown (million, %) by Region 2025 & 2033
- Figure 2: North America Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Treatment Type 2025 & 2033
- Figure 3: North America Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 4: North America Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Country 2025 & 2033
- Figure 5: North America Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 6: Europe Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Treatment Type 2025 & 2033
- Figure 7: Europe Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 8: Europe Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Country 2025 & 2033
- Figure 9: Europe Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 10: Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Treatment Type 2025 & 2033
- Figure 11: Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 12: Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Country 2025 & 2033
- Figure 13: Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 14: Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Treatment Type 2025 & 2033
- Figure 15: Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 16: Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Country 2025 & 2033
- Figure 17: Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 18: South America Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Treatment Type 2025 & 2033
- Figure 19: South America Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 20: South America Chronic Myelogenous Leukemia Treatment Industry Revenue (million), by Country 2025 & 2033
- Figure 21: South America Chronic Myelogenous Leukemia Treatment Industry Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 2: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Region 2020 & 2033
- Table 3: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 4: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Country 2020 & 2033
- Table 5: United States Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 6: Canada Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 7: Mexico Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 8: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 9: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Country 2020 & 2033
- Table 10: Germany Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 11: United Kingdom Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 12: France Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 13: Italy Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 14: Spain Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 15: Rest of Europe Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 16: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 17: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Country 2020 & 2033
- Table 18: China Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 19: Japan Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 20: India Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 21: Australia Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 22: South Korea Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 23: Rest of Asia Pacific Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 24: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 25: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Country 2020 & 2033
- Table 26: GCC Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 27: South Africa Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 28: Rest of Middle East and Africa Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 29: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Treatment Type 2020 & 2033
- Table 30: Global Chronic Myelogenous Leukemia Treatment Industry Revenue million Forecast, by Country 2020 & 2033
- Table 31: Brazil Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 32: Argentina Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 33: Rest of South America Chronic Myelogenous Leukemia Treatment Industry Revenue (million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Chronic Myelogenous Leukemia Treatment Industry?
The projected CAGR is approximately 5.6%.
2. Which companies are prominent players in the Chronic Myelogenous Leukemia Treatment Industry?
Key companies in the market include Fresenius Kabi AG*List Not Exhaustive, Viatris Inc (Mylan), Boehringer Ingelheim International GmbH, Sanofi, Novartis AG, Cipla Inc (Cipla USA Inc ), Bristol-Myers Squibb Co, Merck & Co Inc, F Hoffmann-La Roche Ltd, Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd, Accord Healthcare Inc, Amneal Pharmaceuticals LLC, Pfizer Inc.
3. What are the main segments of the Chronic Myelogenous Leukemia Treatment Industry?
The market segments include Treatment Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 9264.8 million as of 2022.
5. What are some drivers contributing to market growth?
High Incidence and Prevalence of Chronic Myeloid Leukemia; Advancement in Drug Development; Increasing Investments in Research and Development.
6. What are the notable trends driving market growth?
The Chemotherapy Segment is Expected to Witness Significant Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
Side effects Associated with Chemotherapy; Stringent Regulations on Drugs.
8. Can you provide examples of recent developments in the market?
In July 2022, the Center for Drug Evaluation (CDE) of China of the National Medical Products Administration (NMPA) accepted and granted Priority Review designation to a New Drug Application (NDA) submitted by Innovent Biologics, Inc. and Ascentage Pharma that will support the full approval of olverembatinib in patients with chronic-phase chronic myeloid leukemia (CML-CP) who are resistant and/or intolerant of first- and second-generation tyrosine kinase inhibitors (TKIs).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Chronic Myelogenous Leukemia Treatment Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Chronic Myelogenous Leukemia Treatment Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Chronic Myelogenous Leukemia Treatment Industry?
To stay informed about further developments, trends, and reports in the Chronic Myelogenous Leukemia Treatment Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


