Key Insights
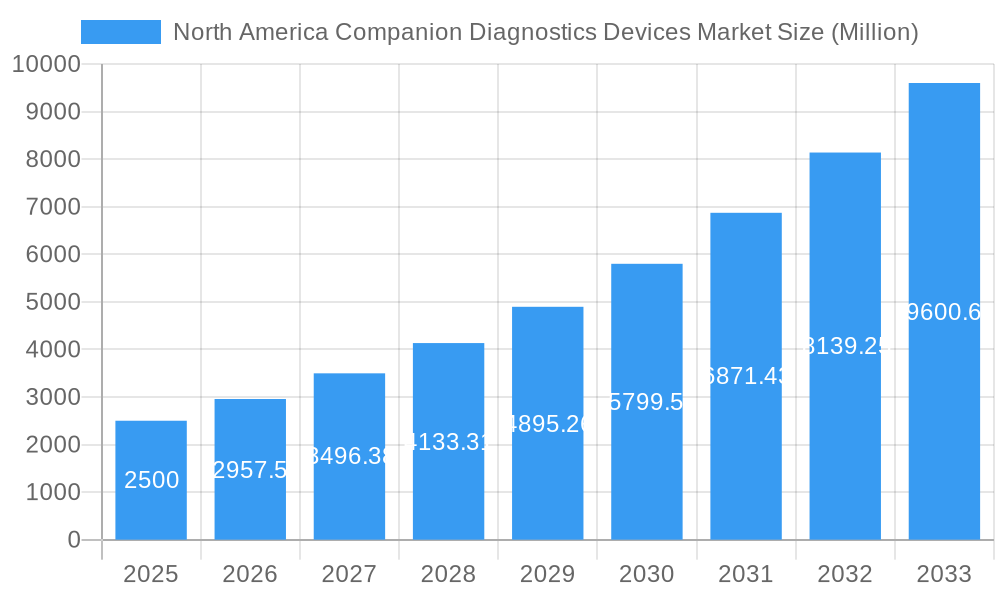
The North American companion diagnostics devices market, valued at $2.5 billion in 2025, is projected to experience robust growth, driven by a compound annual growth rate (CAGR) of 18.50% from 2025 to 2033. This expansion is fueled by several key factors. The rising prevalence of cancer, particularly lung, breast, colorectal, leukemia, and melanoma, necessitates accurate and personalized treatment strategies. Companion diagnostics play a crucial role in this process by guiding therapy selection, ensuring optimal patient outcomes, and improving overall treatment efficacy. Technological advancements, including the increasing adoption of sophisticated techniques like next-generation sequencing (NGS), polymerase chain reaction (PCR), and immunohistochemistry (IHC), are further propelling market growth. These technologies offer enhanced sensitivity and specificity, allowing for earlier and more precise diagnosis, thereby improving patient prognosis and reducing healthcare costs associated with ineffective treatments. The strong presence of major players like Labcorp, Novartis, Biomerieux, and Thermo Fisher Scientific within the North American market contributes significantly to the market's dynamism and innovation. Furthermore, increased investments in research and development and growing collaborations between diagnostic companies and pharmaceutical firms are fostering the development of new and improved companion diagnostic devices.
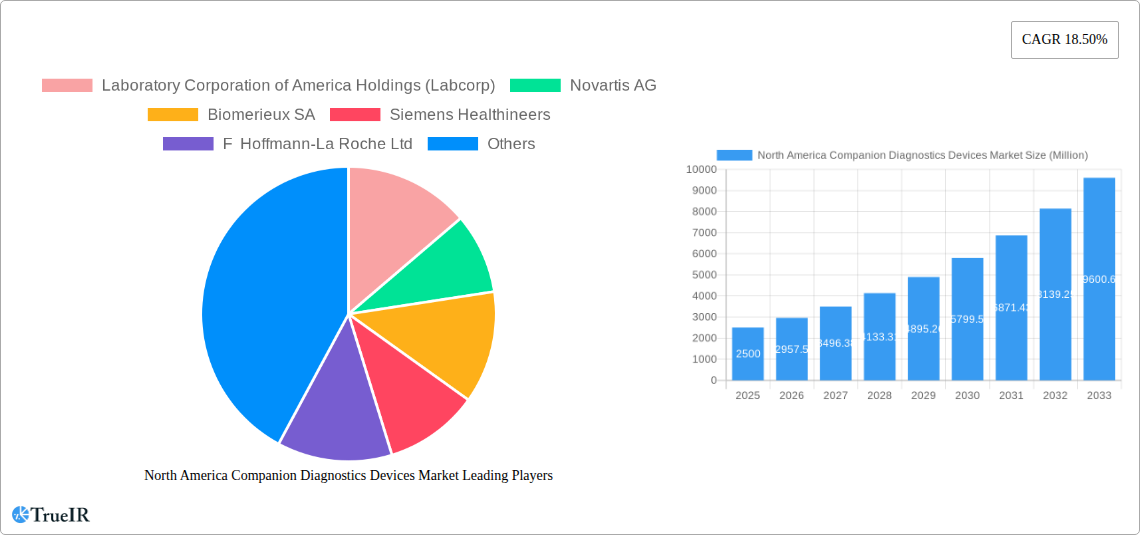
North America Companion Diagnostics Devices Market Market Size (In Billion)
The market segmentation reveals a diverse landscape, with immunohistochemistry (IHC) and PCR technologies currently dominating the market. However, rapid technological advancements in areas such as next-generation sequencing (NGS) are expected to significantly alter the market share distribution over the forecast period. The strong regulatory support within North America, facilitating faster approvals for new companion diagnostic devices, further enhances market attractiveness. While factors such as high costs associated with some advanced technologies and reimbursement challenges might pose some restraints, the overall market trajectory strongly indicates a positive outlook for the North American companion diagnostics devices market in the coming years. The continued focus on personalized medicine and precision oncology will undoubtedly further fuel the market’s growth trajectory.
North America Companion Diagnostics Devices Market Company Market Share
This in-depth report provides a comprehensive analysis of the North America companion diagnostics devices market, encompassing market size, growth projections, key segments, competitive landscape, and future outlook. The study period spans from 2019 to 2033, with 2025 serving as the base and estimated year. The report leverages extensive data and insights to offer a valuable resource for industry stakeholders, investors, and researchers. This detailed analysis incorporates real-world data and avoids the use of placeholders.
North America Companion Diagnostics Devices Market Structure & Competitive Landscape
The North America companion diagnostics devices market is characterized by a moderately concentrated structure with a few major players dominating the landscape. Key players such as Laboratory Corporation of America Holdings (Labcorp), Novartis AG, Biomerieux SA, Siemens Healthineers, F Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc, Abbott Laboratories, ARUP Laboratories, Agilent Technologies Inc, Qiagen NV, Amgen Inc, and Danaher Corporation (Beckman Coulter Inc) contribute significantly to the market's overall value. However, the market also hosts several smaller, specialized companies focusing on niche segments.
- Market Concentration: The Herfindahl-Hirschman Index (HHI) for the market in 2024 is estimated at xx, indicating a moderately concentrated structure.
- Innovation Drivers: Ongoing advancements in genomics, molecular diagnostics, and AI/ML are key drivers of innovation, leading to the development of more precise and efficient companion diagnostics.
- Regulatory Impacts: Stringent regulatory approvals (e.g., FDA approvals) influence market entry and growth, necessitating substantial investment in clinical trials and regulatory compliance.
- Product Substitutes: While direct substitutes are limited, alternative diagnostic methods (e.g., traditional pathology) may influence market penetration.
- End-User Segmentation: Major end-users include hospitals, clinical laboratories, and specialized diagnostic centers. The growth of these facilities directly correlates with the market’s expansion.
- M&A Trends: The market has witnessed xx mergers and acquisitions in the past five years, with larger companies consolidating their market share through strategic acquisitions of smaller, specialized firms. This trend is expected to continue, further shaping the competitive dynamics.
North America Companion Diagnostics Devices Market Market Trends & Opportunities
The North America companion diagnostics devices market exhibits robust growth, driven by rising cancer incidence, increasing demand for personalized medicine, and technological advancements. The market size, valued at $xx Million in 2024, is projected to reach $xx Million by 2033, exhibiting a CAGR of xx% during the forecast period (2025-2033). This growth is fueled by several key trends:
- Technological Shifts: The adoption of advanced technologies like next-generation sequencing (NGS), digital PCR, and microfluidics is enhancing diagnostic accuracy and efficiency, driving market expansion.
- Consumer Preferences: Patients are increasingly demanding personalized medicine approaches, leading to higher demand for companion diagnostics that guide targeted therapies.
- Competitive Dynamics: The market is witnessing increased competition, with both established players and emerging companies vying for market share through product innovation and strategic partnerships.
- Market Penetration: The penetration rate of companion diagnostics is still relatively low compared to the total number of cancer diagnoses, presenting significant untapped potential for market expansion. Increased awareness, coupled with technological advancements, is anticipated to fuel increased penetration.
Dominant Markets & Segments in North America Companion Diagnostics Devices Market
The North America companion diagnostics market is dominated by the United States, accounting for the largest share of market revenue. Within the technology segment, Polymerase Chain Reaction (PCR) and Immunohistochemistry (IHC) hold significant market share, driven by their established usage and widespread availability. In terms of indication, lung cancer, breast cancer, and colorectal cancer are the leading segments, reflecting the high prevalence of these cancers and the availability of targeted therapies.
Key Growth Drivers:
- Technological Advancements: Continuous innovation in PCR, IHC, NGS, and other technologies is a key growth driver.
- Increased Cancer Prevalence: The rising incidence of cancer is a significant factor driving market expansion.
- Government Funding and Initiatives: Government investments in research and development of companion diagnostics accelerate the market's growth.
- Strong Healthcare Infrastructure: The advanced healthcare infrastructure in North America is vital for the market’s development.
- Growing Adoption of Personalized Medicine: The shift towards individualized therapies fuels demand.
North America Companion Diagnostics Devices Market Product Analysis
The market offers a diverse range of products, including PCR-based assays, IHC kits, ISH probes, NGS platforms, and other companion diagnostic devices. Technological advancements are driving the development of more sophisticated and accurate devices, improving diagnostic capabilities, enhancing patient outcomes, and leading to greater adoption by healthcare professionals. This trend towards enhanced precision and efficiency is a key competitive advantage for market players.
Key Drivers, Barriers & Challenges in North America Companion Diagnostics Devices Market
Key Drivers:
The market is propelled by technological innovations, increasing cancer prevalence, and the rising adoption of targeted therapies guided by companion diagnostics. Government funding for research and development further accelerates growth.
Key Challenges & Restraints:
High development costs, stringent regulatory approvals, reimbursement challenges, and competition from existing diagnostic methods create significant barriers. Supply chain disruptions and potential manufacturing bottlenecks may also impact market growth. The estimated impact of these restraints on the market's growth is approximately xx% during the forecast period.
Growth Drivers in the North America Companion Diagnostics Devices Market Market
Technological advancements, rising cancer incidence, personalized medicine adoption, government funding for R&D, and strong healthcare infrastructure are key drivers.
Challenges Impacting North America Companion Diagnostics Devices Market Growth
High development costs, stringent regulatory processes, reimbursement hurdles, competition from established diagnostics, and supply chain issues pose significant challenges.
Key Players Shaping the North America Companion Diagnostics Devices Market Market
Significant North America Companion Diagnostics Devices Market Industry Milestones
- December 2022: QIAGEN received FDA approval for its therascreen KRAS RGQ PCR kit, a companion diagnostic for Mirati Therapeutics' KRAZATI (adagrasib) in non-small cell lung cancer (NSCLC). This significantly expanded QIAGEN's market reach within the oncology segment.
- January 2023: QIAGEN partnered exclusively with Helix to advance companion diagnostics for hereditary diseases (cardiovascular, metabolic, neurodegenerative, autoimmune). This strategic move broadened QIAGEN’s portfolio and market reach beyond oncology.
Future Outlook for North America Companion Diagnostics Devices Market Market
The North America companion diagnostics devices market is poised for continued strong growth, driven by technological advancements, increasing demand for personalized medicine, and a rising prevalence of cancer. Strategic partnerships, product innovation, and expansion into new therapeutic areas will be crucial for market players to capitalize on the significant growth potential within this dynamic sector.
North America Companion Diagnostics Devices Market Segmentation
-
1. Technology
- 1.1. Immunohistochemistry (IHC)
- 1.2. Polymerase Chain Reaction (PCR)
- 1.3. In-situ Hybridization (ISH)
- 1.4. Real-time PCR (RT-PCR)
- 1.5. Gene Sequencing
- 1.6. Other Technologies
-
2. Indication
- 2.1. Lung Cancer
- 2.2. Breast Cancer
- 2.3. Colorectal Cancer
- 2.4. Leukemia
- 2.5. Melanoma
- 2.6. Other Indications
-
3. Geography
-
3.1. North America
- 3.1.1. United States
- 3.1.2. Canada
- 3.1.3. Mexico
-
3.1. North America
North America Companion Diagnostics Devices Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
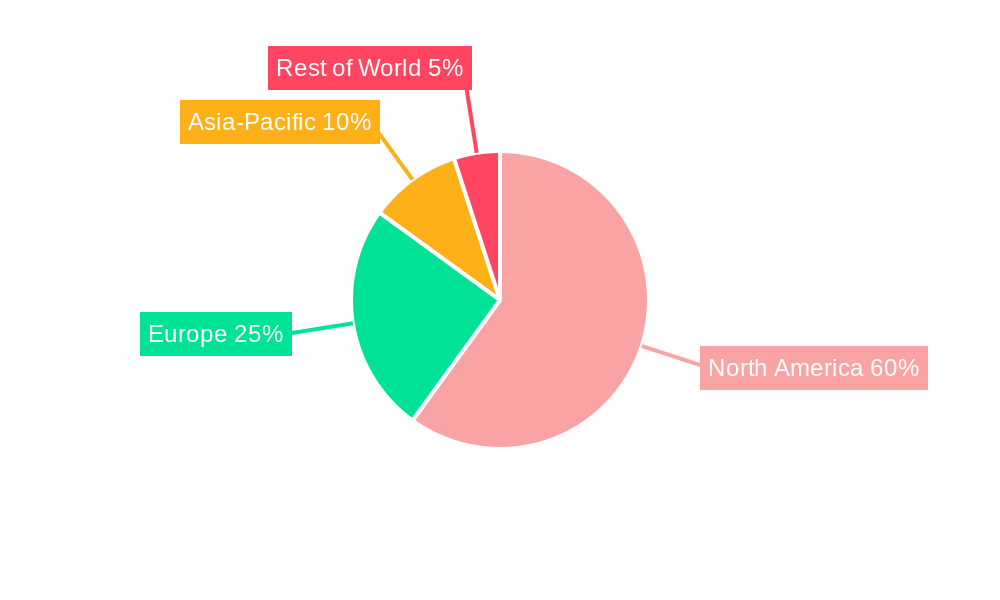
North America Companion Diagnostics Devices Market Regional Market Share
Geographic Coverage of North America Companion Diagnostics Devices Market
North America Companion Diagnostics Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 18.50% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Demand and Awareness for Personalized Medicine and Targeted Therapy; Increasing Cases of Adverse Drug Reactions; Technological Advancements in the Devices
- 3.3. Market Restrains
- 3.3.1. High Cost of Drug Development and Associated Clinical Trials
- 3.4. Market Trends
- 3.4.1. The In-situ Hybridization (ISH) Segment is Expected to Exhibit the Fastest Growth Rate over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. North America Companion Diagnostics Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 5.1.1. Immunohistochemistry (IHC)
- 5.1.2. Polymerase Chain Reaction (PCR)
- 5.1.3. In-situ Hybridization (ISH)
- 5.1.4. Real-time PCR (RT-PCR)
- 5.1.5. Gene Sequencing
- 5.1.6. Other Technologies
- 5.2. Market Analysis, Insights and Forecast - by Indication
- 5.2.1. Lung Cancer
- 5.2.2. Breast Cancer
- 5.2.3. Colorectal Cancer
- 5.2.4. Leukemia
- 5.2.5. Melanoma
- 5.2.6. Other Indications
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.3.1. North America
- 5.3.1.1. United States
- 5.3.1.2. Canada
- 5.3.1.3. Mexico
- 5.3.1. North America
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Laboratory Corporation of America Holdings (Labcorp)
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Novartis AG
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Biomerieux SA
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Siemens Healthineers
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 F Hoffmann-La Roche Ltd
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Thermo Fisher Scientific Inc
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Abbott Laboratories
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 ARUP Laboratories
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Agilent Technologies Inc
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Qiagen NV
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.11 Amgen Inc *List Not Exhaustive
- 6.2.11.1. Overview
- 6.2.11.2. Products
- 6.2.11.3. SWOT Analysis
- 6.2.11.4. Recent Developments
- 6.2.11.5. Financials (Based on Availability)
- 6.2.12 Danaher Corporation (Beckman Coulter Inc )
- 6.2.12.1. Overview
- 6.2.12.2. Products
- 6.2.12.3. SWOT Analysis
- 6.2.12.4. Recent Developments
- 6.2.12.5. Financials (Based on Availability)
- 6.2.1 Laboratory Corporation of America Holdings (Labcorp)
List of Figures
- Figure 1: North America Companion Diagnostics Devices Market Revenue Breakdown (Million, %) by Product 2025 & 2033
- Figure 2: North America Companion Diagnostics Devices Market Share (%) by Company 2025
List of Tables
- Table 1: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 2: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Technology 2020 & 2033
- Table 3: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 4: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Indication 2020 & 2033
- Table 5: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 6: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Geography 2020 & 2033
- Table 7: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Region 2020 & 2033
- Table 8: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Region 2020 & 2033
- Table 9: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 10: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Technology 2020 & 2033
- Table 11: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 12: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Indication 2020 & 2033
- Table 13: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 14: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Geography 2020 & 2033
- Table 15: North America Companion Diagnostics Devices Market Revenue Million Forecast, by Country 2020 & 2033
- Table 16: North America Companion Diagnostics Devices Market Volume K Units Forecast, by Country 2020 & 2033
- Table 17: United States North America Companion Diagnostics Devices Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: United States North America Companion Diagnostics Devices Market Volume (K Units) Forecast, by Application 2020 & 2033
- Table 19: Canada North America Companion Diagnostics Devices Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: Canada North America Companion Diagnostics Devices Market Volume (K Units) Forecast, by Application 2020 & 2033
- Table 21: Mexico North America Companion Diagnostics Devices Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 22: Mexico North America Companion Diagnostics Devices Market Volume (K Units) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the North America Companion Diagnostics Devices Market?
The projected CAGR is approximately 18.50%.
2. Which companies are prominent players in the North America Companion Diagnostics Devices Market?
Key companies in the market include Laboratory Corporation of America Holdings (Labcorp), Novartis AG, Biomerieux SA, Siemens Healthineers, F Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc, Abbott Laboratories, ARUP Laboratories, Agilent Technologies Inc, Qiagen NV, Amgen Inc *List Not Exhaustive, Danaher Corporation (Beckman Coulter Inc ).
3. What are the main segments of the North America Companion Diagnostics Devices Market?
The market segments include Technology, Indication, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 2.5 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Demand and Awareness for Personalized Medicine and Targeted Therapy; Increasing Cases of Adverse Drug Reactions; Technological Advancements in the Devices.
6. What are the notable trends driving market growth?
The In-situ Hybridization (ISH) Segment is Expected to Exhibit the Fastest Growth Rate over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Drug Development and Associated Clinical Trials.
8. Can you provide examples of recent developments in the market?
In January 2023, QIAGEN entered into an exclusive strategic partnership with Helix o advance companion diagnostics for hereditary diseases such as cardiovascular, metabolic, neurodegenerative, auto-immune diseases, and others. Under the terms of the agreement, QIAGEN will be the exclusive marketing and contracting partner in the United States for Helix's companion diagnostic services.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "North America Companion Diagnostics Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the North America Companion Diagnostics Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the North America Companion Diagnostics Devices Market?
To stay informed about further developments, trends, and reports in the North America Companion Diagnostics Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
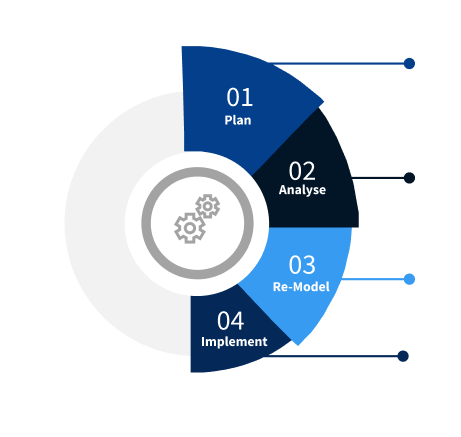
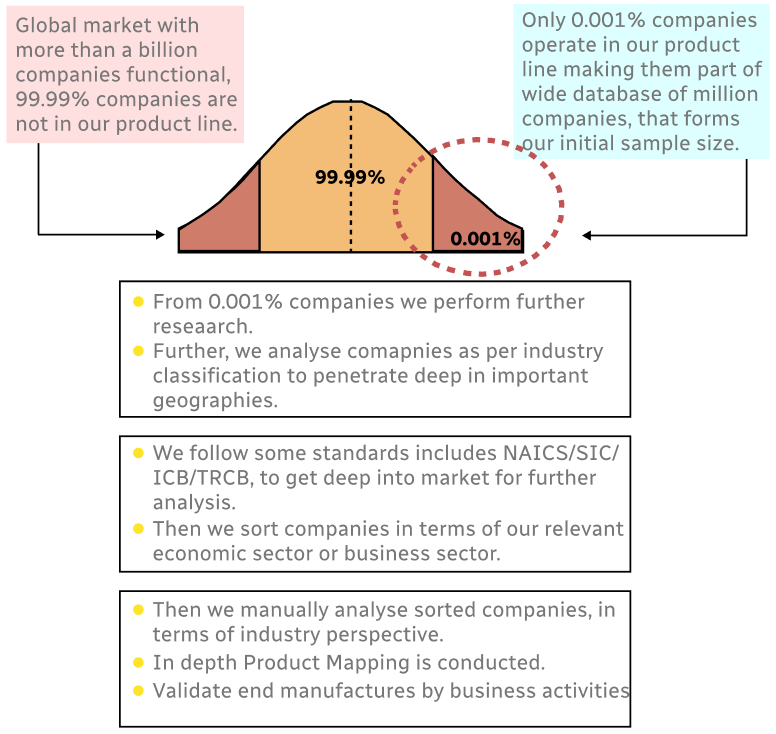
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


