Key Insights
The Pediatric Neuroblastoma Treatment market is projected for substantial growth, reaching an estimated $13.51 billion by 2033, driven by a robust Compound Annual Growth Rate (CAGR) of 9.1% from a base year of 2025. This expansion is fueled by advancements in therapeutic approaches, particularly the increasing adoption of immunotherapy alongside established chemotherapy regimens. Rising global incidence of neuroblastoma in children, coupled with a greater emphasis on early diagnosis and improved treatment outcomes, are key market accelerators. The development of novel drug formulations and targeted therapies is crucial for enhancing treatment efficacy and patient survival rates. Increased healthcare expenditure on pediatric oncology and growing awareness among parents and healthcare professionals about neuroblastoma symptoms and available treatments further contribute to this positive market trajectory.
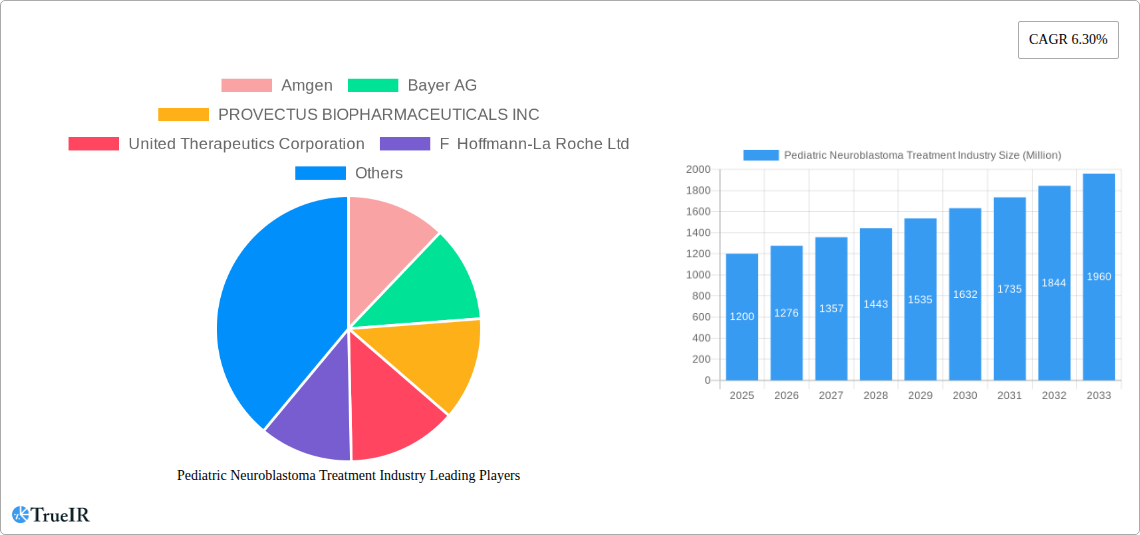
Pediatric Neuroblastoma Treatment Industry Market Size (In Billion)

The market landscape features a dynamic interplay between established pharmaceutical giants and innovative biotechnology firms developing cutting-edge treatments. Key players are investing heavily in research and development, focusing on optimizing existing therapies and exploring new frontiers in neuroblastoma treatment. Strategic distribution channels, with hospital pharmacies leading due to the critical nature of neuroblastoma care, are essential. While chemotherapy remains a cornerstone, the rising prominence of immunotherapy signifies a paradigm shift towards more personalized and less toxic treatment options. Challenges such as the high cost of novel therapies, the need for specialized infrastructure, and the complexities of pediatric clinical trials present ongoing restraints. Despite these hurdles, persistent demand for more effective and less debilitating treatments fuels ongoing innovation and market growth.
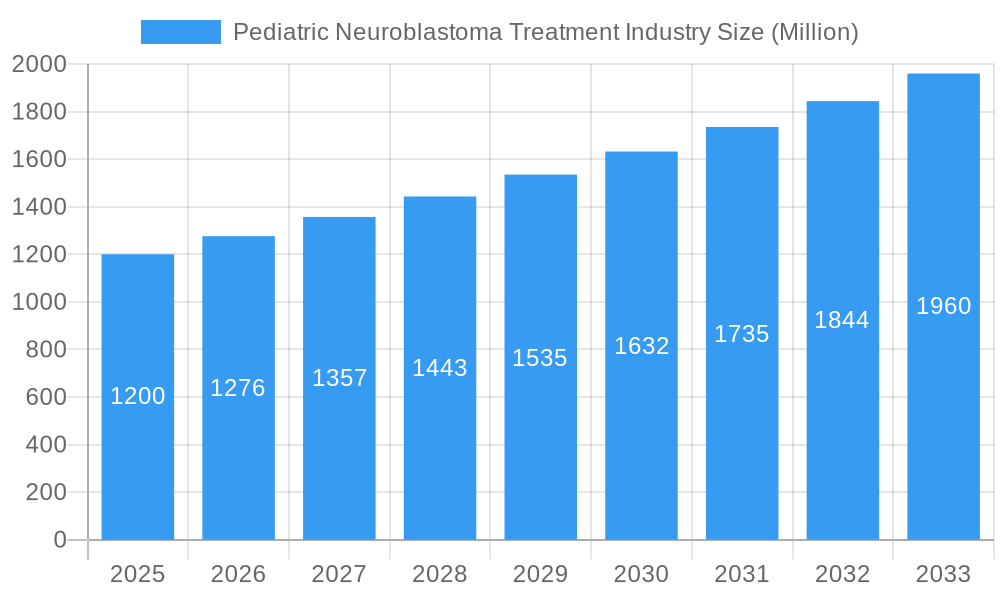
Pediatric Neuroblastoma Treatment Industry Company Market Share

This report delivers in-depth analysis of the global pediatric neuroblastoma treatment market, offering critical insights for stakeholders from 2019 to 2033. With a base year of 2025 and a forecast period extending to 2033, this study examines market structure, key trends, dominant segments, product innovations, growth drivers, barriers, and the competitive landscape. Leveraging high-volume keywords such as "pediatric neuroblastoma treatment," "neuroblastoma therapy," "pediatric oncology drugs," and "cancer immunotherapy children," this report aims to enhance SEO rankings and provide actionable intelligence for pharmaceutical companies, research institutions, investors, and healthcare providers.
Pediatric Neuroblastoma Treatment Industry Market Structure & Competitive Landscape
The pediatric neuroblastoma treatment market exhibits a moderately concentrated structure, driven by a combination of established pharmaceutical giants and emerging biopharmaceutical innovators. Innovation in novel therapeutic modalities, particularly in immunotherapy and targeted therapies, serves as a primary driver, pushing the boundaries of treatment efficacy. Regulatory bodies play a crucial role, with stringent approval processes balancing patient safety and market access. The threat of product substitutes, while present in the form of alternative cancer treatments, is mitigated by the specific nature of neuroblastoma and the ongoing development of tailored solutions. End-user segmentation predominantly includes pediatric oncology centers and specialized cancer treatment facilities. Merger and acquisition (M&A) activity is observed as companies seek to consolidate portfolios, acquire promising pipeline assets, and expand their market presence. Historical M&A volumes are estimated in the hundreds of millions, with strategic acquisitions of early-stage biotech firms focused on neuroblastoma therapies being a recurring theme. Concentration ratios for top players are estimated to be between 40-50%.
Pediatric Neuroblastoma Treatment Industry Market Trends & Opportunities
The pediatric neuroblastoma treatment market is poised for significant expansion, projected to reach a valuation of over $2,000 million by 2033. This growth is underpinned by a Compound Annual Growth Rate (CAGR) of approximately 8.5% throughout the forecast period. Key trends shaping the market include the increasing adoption of personalized medicine approaches, driven by advancements in genomic profiling and biomarker discovery, allowing for more precise and effective treatment selection. Immunotherapy, with its potential for durable responses and reduced toxicity, is emerging as a cornerstone of pediatric neuroblastoma treatment, moving beyond traditional chemotherapy. Technological shifts are evident in the development of novel drug delivery systems, combination therapies, and early diagnostic tools. Consumer preferences are increasingly focused on treatments that minimize long-term side effects and improve the quality of life for young patients. Competitive dynamics are intensifying, with ongoing research and development efforts focused on overcoming treatment resistance and addressing relapsed or refractory cases. Market penetration for advanced therapies like immunotherapy is steadily increasing, projected to surpass 30% by 2033, presenting substantial opportunities for companies at the forefront of innovation. The growing understanding of neuroblastoma biology, coupled with expanded access to advanced treatment centers, is further fueling market growth.
Dominant Markets & Segments in Pediatric Neuroblastoma Treatment Industry
North America currently leads the pediatric neuroblastoma treatment market, driven by a robust healthcare infrastructure, high R&D investment, and a strong presence of leading pharmaceutical and biotechnology companies. The United States, in particular, represents a significant market share due to a large patient population and advanced clinical trial capabilities. Within treatment types, Immunotherapy is emerging as a dominant segment, showcasing rapid growth and promising clinical outcomes.
- Key Growth Drivers for Immunotherapy:
- Advancements in CAR-T cell therapy and antibody-drug conjugates (ADCs) are revolutionizing treatment paradigms.
- Increased understanding of the tumor microenvironment enabling the development of more effective immunomodulatory agents.
- Supportive government initiatives and funding for rare pediatric diseases research.
- Positive clinical trial data demonstrating superior efficacy and tolerability compared to conventional therapies.
The Hospital Pharmacies distribution channel also holds a dominant position, reflecting the complex and specialized nature of neuroblastoma treatment, which typically requires inpatient administration and multidisciplinary care coordination.
- Dominance of Hospital Pharmacies:
- Specialized compounding and administration of potent therapeutic agents.
- Integrated care models facilitating seamless patient management.
- Access to a wider range of investigational drugs through clinical trials conducted within hospital settings.
- Expertise in managing treatment-related toxicities and side effects.
Other treatment types, including advanced chemotherapy regimens and targeted therapies, continue to play a vital role, contributing significantly to the overall market revenue. While retail pharmacies cater to some outpatient aspects, the critical nature of neuroblastoma care solidifies the dominance of hospital-based distribution.
Pediatric Neuroblastoma Treatment Industry Product Analysis
The pediatric neuroblastoma treatment landscape is characterized by a surge in product innovations aimed at enhancing efficacy and minimizing toxicity. Advancements include novel immunotherapy agents, such as bispecific antibodies and CAR-T cell therapies, designed to elicit potent anti-tumor responses. Targeted therapies, including kinase inhibitors and differentiation agents, are being developed to address specific genetic mutations within neuroblastoma cells. These innovations offer competitive advantages through improved response rates, reduced side effects, and the potential for long-term remission, addressing unmet needs in refractory and relapsed cases.
Key Drivers, Barriers & Challenges in Pediatric Neuroblastoma Treatment Industry
Key Drivers:
- Technological Advancements: Breakthroughs in genomics, immunotherapy, and targeted drug development are fueling market growth. The development of novel antibody-drug conjugates and personalized cell therapies offers significant potential.
- Increasing Incidence and Awareness: A gradual rise in neuroblastoma diagnoses, coupled with enhanced global awareness, drives demand for effective treatments.
- Regulatory Support: Expedited review pathways and designations for rare pediatric diseases (RPDs) by regulatory bodies accelerate drug approvals.
- Investment in R&D: Robust funding from government agencies and private investors supports the development of innovative therapies.
Key Barriers & Challenges:
- High Cost of Treatment: Advanced therapies, particularly immunotherapies, are exceptionally expensive, posing significant access challenges.
- Regulatory Hurdles: Stringent clinical trial requirements and complex approval processes can delay market entry for new drugs.
- Limited Patient Pool: Neuroblastoma, while serious, remains a relatively rare disease, impacting the economics of large-scale drug development.
- Treatment Resistance: The development of resistance to existing therapies remains a significant challenge, necessitating continuous innovation. Supply chain complexities for specialized biologics can also present issues, with potential delays estimated at 5-10%.
Growth Drivers in the Pediatric Neuroblastoma Treatment Industry Market
Growth in the pediatric neuroblastoma treatment market is primarily propelled by groundbreaking advancements in immunotherapy, with novel approaches like CAR-T therapy and bispecific antibodies demonstrating significant promise. The increasing focus on precision medicine, driven by a deeper understanding of neuroblastoma's genetic landscape, allows for the development of highly targeted therapies, improving treatment outcomes. Regulatory incentives, such as Orphan Drug designations and Fast Track designations, expedite the development and approval of novel treatments for this rare pediatric disease, encouraging pharmaceutical investment. Furthermore, increased global R&D expenditure by both established pharmaceutical giants and agile biotechnology firms is continuously expanding the pipeline of innovative therapeutic options, creating a dynamic environment for growth.
Challenges Impacting Pediatric Neuroblastoma Treatment Industry Growth
The pediatric neuroblastoma treatment market faces several significant challenges. The exorbitant cost associated with cutting-edge treatments, particularly advanced immunotherapies, creates substantial affordability and accessibility issues for many healthcare systems and patient families, estimated to increase treatment costs by 20-30% per patient. Stringent and lengthy regulatory pathways, despite existing incentives, can still pose considerable hurdles to timely drug approvals. Therapeutic resistance remains a persistent concern, with neuroblastoma cells often developing mechanisms to evade treatment over time. Additionally, the limited patient population inherent to a rare pediatric cancer can impact the commercial viability of developing and manufacturing highly specialized therapies, potentially leading to supply chain vulnerabilities and increased per-unit production costs.
Key Players Shaping the Pediatric Neuroblastoma Treatment Industry Market
- Amgen
- Bayer AG
- PROVECTUS BIOPHARMACEUTICALS INC
- United Therapeutics Corporation
- F Hoffmann-La Roche Ltd
- Eli Lilly and Company
- APEIRON Biologics AG
- Sartorius AG
- Y-mAbs Therapeutics Inc
- CELLECTAR BIOSCIENCES INC
- MacroGenics Inc
- Baxter International
- Pfizer Inc
Significant Pediatric Neuroblastoma Treatment Industry Industry Milestones
- October 2022: Kyushu University sponsored a clinical trial titled "Clinical Trial of GAIA-102 for Refractory/Relapse Neuroblastomas or Malignant Pediatric Solid Tumors With Lung Metastases" to evaluate the safety of GAIA-102.
- February 2022: The United States Food and Drug Administration granted a Fast Track Designation (FTD) to the targeted inhibitor alrizomadlin (APG-115) for the treatment of neuroblastoma as a rare pediatric disease (RPD).
Future Outlook for Pediatric Neuroblastoma Treatment Industry Market
The future outlook for the pediatric neuroblastoma treatment market is exceptionally promising, driven by relentless innovation and a growing understanding of the disease. Strategic opportunities lie in the further development and widespread adoption of combination immunotherapies, harnessing the synergistic potential of different treatment modalities. The expansion of early diagnostic tools and liquid biopsy techniques will enable earlier intervention and more personalized treatment strategies. Continued investment in pediatric-focused research and development, coupled with supportive regulatory frameworks, will foster a robust pipeline of novel agents. The market is expected to witness substantial growth, with a projected increase in the overall market size by over $1,500 million within the forecast period, fueled by the successful translation of promising clinical candidates into approved therapies and improved patient outcomes.
Pediatric Neuroblastoma Treatment Industry Segmentation
-
1. Treatment Type
- 1.1. Chemotherpy
- 1.2. Immunotherapy
- 1.3. Other Treatment Types
-
2. Distribution Channel
- 2.1. Hospital Pharmacies
- 2.2. Retail Pharmacies
Pediatric Neuroblastoma Treatment Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
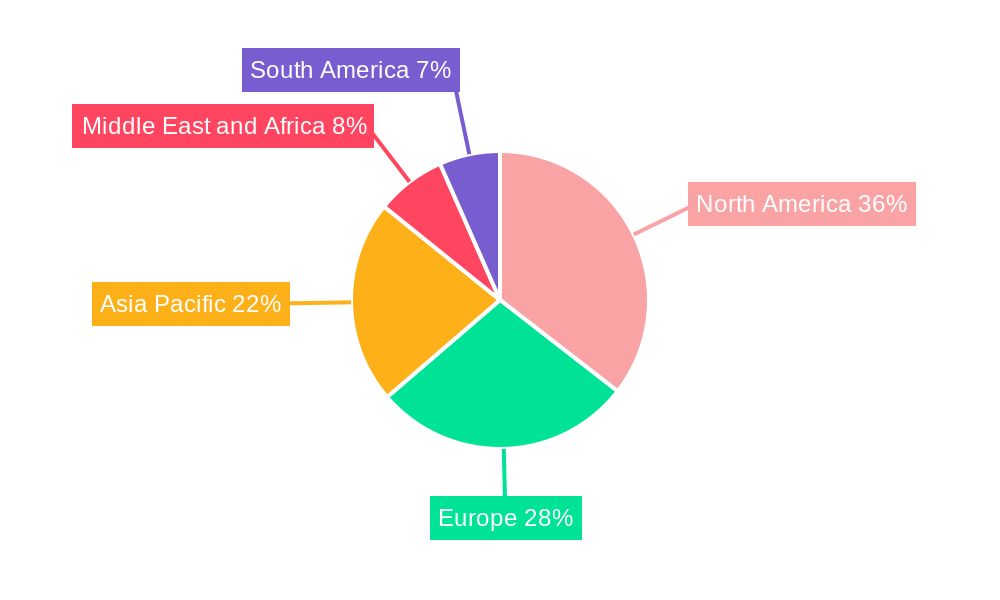
Pediatric Neuroblastoma Treatment Industry Regional Market Share

Geographic Coverage of Pediatric Neuroblastoma Treatment Industry
Pediatric Neuroblastoma Treatment Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 9.1% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Burden Of Pediatric Neuroblastoma Coupled With Increasing R&D; Rise in Awareness of Available Treatment For Cancer
- 3.3. Market Restrains
- 3.3.1. High Cost And Side Effects Associated With Cancer Therapy
- 3.4. Market Trends
- 3.4.1. TheChemotherapy Segment is Anticipated to Witness a Growth in the Pediatric Neuroblastoma Treatment Market Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Treatment Type
- 5.1.1. Chemotherpy
- 5.1.2. Immunotherapy
- 5.1.3. Other Treatment Types
- 5.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 5.2.1. Hospital Pharmacies
- 5.2.2. Retail Pharmacies
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East and Africa
- 5.3.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Treatment Type
- 6. North America Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Treatment Type
- 6.1.1. Chemotherpy
- 6.1.2. Immunotherapy
- 6.1.3. Other Treatment Types
- 6.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 6.2.1. Hospital Pharmacies
- 6.2.2. Retail Pharmacies
- 6.1. Market Analysis, Insights and Forecast - by Treatment Type
- 7. Europe Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Treatment Type
- 7.1.1. Chemotherpy
- 7.1.2. Immunotherapy
- 7.1.3. Other Treatment Types
- 7.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 7.2.1. Hospital Pharmacies
- 7.2.2. Retail Pharmacies
- 7.1. Market Analysis, Insights and Forecast - by Treatment Type
- 8. Asia Pacific Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Treatment Type
- 8.1.1. Chemotherpy
- 8.1.2. Immunotherapy
- 8.1.3. Other Treatment Types
- 8.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 8.2.1. Hospital Pharmacies
- 8.2.2. Retail Pharmacies
- 8.1. Market Analysis, Insights and Forecast - by Treatment Type
- 9. Middle East and Africa Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Treatment Type
- 9.1.1. Chemotherpy
- 9.1.2. Immunotherapy
- 9.1.3. Other Treatment Types
- 9.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 9.2.1. Hospital Pharmacies
- 9.2.2. Retail Pharmacies
- 9.1. Market Analysis, Insights and Forecast - by Treatment Type
- 10. South America Pediatric Neuroblastoma Treatment Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Treatment Type
- 10.1.1. Chemotherpy
- 10.1.2. Immunotherapy
- 10.1.3. Other Treatment Types
- 10.2. Market Analysis, Insights and Forecast - by Distribution Channel
- 10.2.1. Hospital Pharmacies
- 10.2.2. Retail Pharmacies
- 10.1. Market Analysis, Insights and Forecast - by Treatment Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Amgen
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Bayer AG
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 PROVECTUS BIOPHARMACEUTICALS INC
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 United Therapeutics Corporation
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 F Hoffmann-La Roche Ltd
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Eli Lilly and Company
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 APEIRON Biologics AG
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Sartorius AG
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Y-mAbs Therapeutics Inc
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 CELLECTAR BIOSCIENCES INC
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 MacroGenics Inc
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Baxter International
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Pfizer Inc
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.1 Amgen
List of Figures
- Figure 1: Global Pediatric Neuroblastoma Treatment Industry Revenue Breakdown (billion, %) by Region 2025 & 2033
- Figure 2: Global Pediatric Neuroblastoma Treatment Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Treatment Type 2025 & 2033
- Figure 4: North America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Treatment Type 2025 & 2033
- Figure 5: North America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 6: North America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Treatment Type 2025 & 2033
- Figure 7: North America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Distribution Channel 2025 & 2033
- Figure 8: North America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Distribution Channel 2025 & 2033
- Figure 9: North America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Distribution Channel 2025 & 2033
- Figure 10: North America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Distribution Channel 2025 & 2033
- Figure 11: North America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Country 2025 & 2033
- Figure 12: North America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Country 2025 & 2033
- Figure 13: North America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 14: North America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Country 2025 & 2033
- Figure 15: Europe Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Treatment Type 2025 & 2033
- Figure 16: Europe Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Treatment Type 2025 & 2033
- Figure 17: Europe Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 18: Europe Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Treatment Type 2025 & 2033
- Figure 19: Europe Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Distribution Channel 2025 & 2033
- Figure 20: Europe Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Distribution Channel 2025 & 2033
- Figure 21: Europe Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Distribution Channel 2025 & 2033
- Figure 22: Europe Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Distribution Channel 2025 & 2033
- Figure 23: Europe Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Country 2025 & 2033
- Figure 24: Europe Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Country 2025 & 2033
- Figure 25: Europe Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 26: Europe Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Country 2025 & 2033
- Figure 27: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Treatment Type 2025 & 2033
- Figure 28: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Treatment Type 2025 & 2033
- Figure 29: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 30: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Treatment Type 2025 & 2033
- Figure 31: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Distribution Channel 2025 & 2033
- Figure 32: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Distribution Channel 2025 & 2033
- Figure 33: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Distribution Channel 2025 & 2033
- Figure 34: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Distribution Channel 2025 & 2033
- Figure 35: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Country 2025 & 2033
- Figure 36: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Country 2025 & 2033
- Figure 37: Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 38: Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Country 2025 & 2033
- Figure 39: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Treatment Type 2025 & 2033
- Figure 40: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Treatment Type 2025 & 2033
- Figure 41: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 42: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Treatment Type 2025 & 2033
- Figure 43: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Distribution Channel 2025 & 2033
- Figure 44: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Distribution Channel 2025 & 2033
- Figure 45: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Distribution Channel 2025 & 2033
- Figure 46: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Distribution Channel 2025 & 2033
- Figure 47: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Country 2025 & 2033
- Figure 48: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: South America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Treatment Type 2025 & 2033
- Figure 52: South America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Treatment Type 2025 & 2033
- Figure 53: South America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Treatment Type 2025 & 2033
- Figure 54: South America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Treatment Type 2025 & 2033
- Figure 55: South America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Distribution Channel 2025 & 2033
- Figure 56: South America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Distribution Channel 2025 & 2033
- Figure 57: South America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Distribution Channel 2025 & 2033
- Figure 58: South America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Distribution Channel 2025 & 2033
- Figure 59: South America Pediatric Neuroblastoma Treatment Industry Revenue (billion), by Country 2025 & 2033
- Figure 60: South America Pediatric Neuroblastoma Treatment Industry Volume (K Unit), by Country 2025 & 2033
- Figure 61: South America Pediatric Neuroblastoma Treatment Industry Revenue Share (%), by Country 2025 & 2033
- Figure 62: South America Pediatric Neuroblastoma Treatment Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 2: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 3: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 4: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 5: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Region 2020 & 2033
- Table 6: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 7: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 8: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 9: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 10: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 11: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 12: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 13: United States Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 14: United States Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 15: Canada Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 16: Canada Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 17: Mexico Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 18: Mexico Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 20: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 21: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 22: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 23: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 24: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: Germany Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 26: Germany Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 27: United Kingdom Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 28: United Kingdom Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 29: France Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 30: France Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 31: Italy Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 32: Italy Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: Spain Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 34: Spain Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: Rest of Europe Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 36: Rest of Europe Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 38: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 39: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 40: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 41: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 42: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 43: China Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 44: China Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 45: Japan Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 46: Japan Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 47: India Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 48: India Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 49: Australia Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 50: Australia Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 51: South Korea Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 52: South Korea Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Rest of Asia Pacific Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 54: Rest of Asia Pacific Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 56: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 57: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 58: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 59: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 60: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 61: GCC Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 62: GCC Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: South Africa Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 64: South Africa Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 65: Rest of Middle East and Africa Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 66: Rest of Middle East and Africa Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 67: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Treatment Type 2020 & 2033
- Table 68: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Treatment Type 2020 & 2033
- Table 69: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Distribution Channel 2020 & 2033
- Table 70: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Distribution Channel 2020 & 2033
- Table 71: Global Pediatric Neuroblastoma Treatment Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 72: Global Pediatric Neuroblastoma Treatment Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 73: Brazil Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 74: Brazil Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Argentina Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 76: Argentina Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Rest of South America Pediatric Neuroblastoma Treatment Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 78: Rest of South America Pediatric Neuroblastoma Treatment Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Pediatric Neuroblastoma Treatment Industry?
The projected CAGR is approximately 9.1%.
2. Which companies are prominent players in the Pediatric Neuroblastoma Treatment Industry?
Key companies in the market include Amgen, Bayer AG, PROVECTUS BIOPHARMACEUTICALS INC, United Therapeutics Corporation, F Hoffmann-La Roche Ltd, Eli Lilly and Company, APEIRON Biologics AG, Sartorius AG, Y-mAbs Therapeutics Inc, CELLECTAR BIOSCIENCES INC, MacroGenics Inc, Baxter International, Pfizer Inc.
3. What are the main segments of the Pediatric Neuroblastoma Treatment Industry?
The market segments include Treatment Type, Distribution Channel.
4. Can you provide details about the market size?
The market size is estimated to be USD 13.51 billion as of 2022.
5. What are some drivers contributing to market growth?
Increasing Burden Of Pediatric Neuroblastoma Coupled With Increasing R&D; Rise in Awareness of Available Treatment For Cancer.
6. What are the notable trends driving market growth?
TheChemotherapy Segment is Anticipated to Witness a Growth in the Pediatric Neuroblastoma Treatment Market Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost And Side Effects Associated With Cancer Therapy.
8. Can you provide examples of recent developments in the market?
In October 2022, Kyushu University sponsored a clinical trial under the title "Clinical Trial of GAIA-102 for Refractory/Relapse Neuroblastomas or Malignant Pediatric Solid Tumors With Lung Metastases" to evaluate the safety of GAIA-102 alone refractory/relapse neuroblastoma or pediatric solid tumors with lung metastases and to decide a recommended dose for Phase II.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Pediatric Neuroblastoma Treatment Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Pediatric Neuroblastoma Treatment Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Pediatric Neuroblastoma Treatment Industry?
To stay informed about further developments, trends, and reports in the Pediatric Neuroblastoma Treatment Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
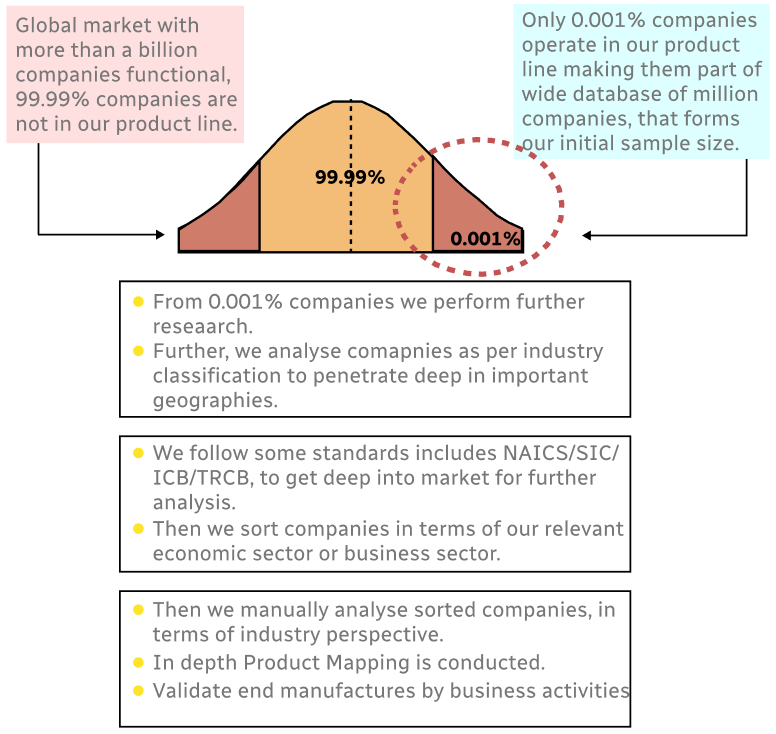
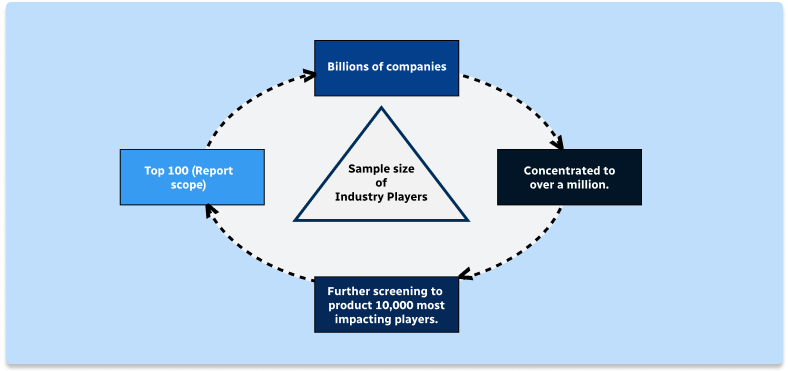
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
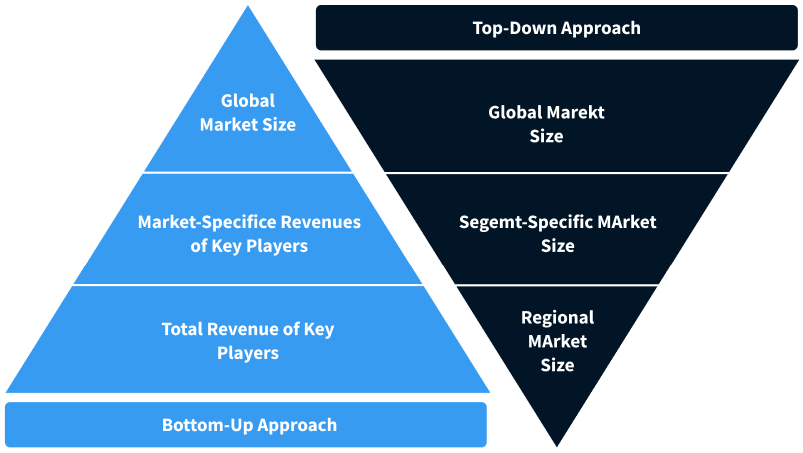
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


