Key Insights
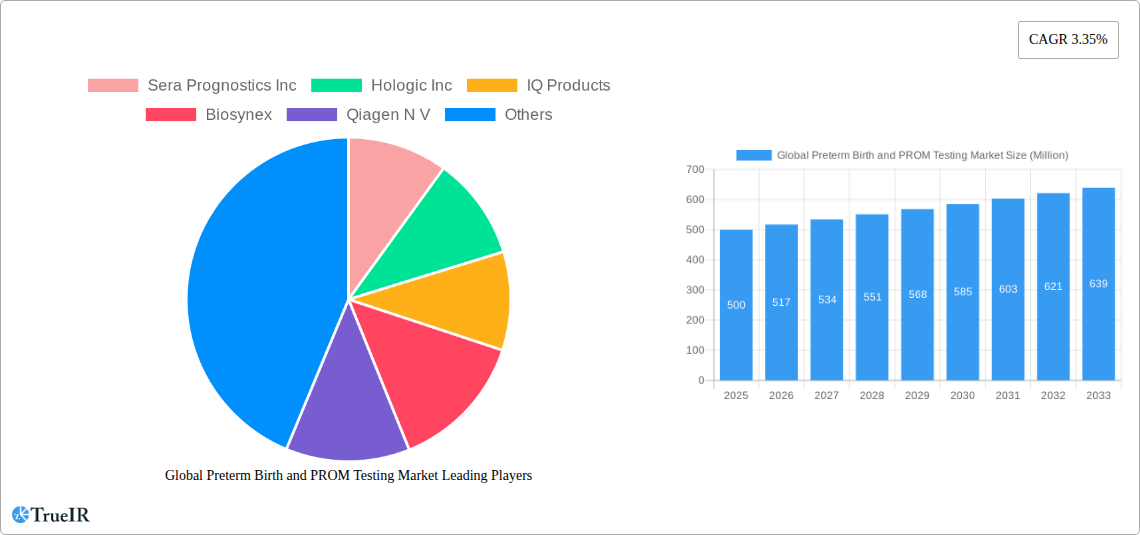
The Global Preterm Birth and PROM Testing Market is projected for significant growth, driven by increasing awareness of prematurity-related complications and the rising incidence of preterm births worldwide. Estimated at approximately \$500 million in 2025, the market is expected to expand at a Compound Annual Growth Rate (CAGR) of 3.35% through 2033. This growth is primarily fueled by advancements in diagnostic technologies, including more accurate ultrasound techniques, sophisticated biochemical markers, and continuous uterine monitoring systems. The escalating healthcare expenditure on maternal and neonatal care, coupled with stringent regulatory frameworks promoting early detection and intervention for preterm labor, are also substantial market accelerators. Furthermore, the increasing prevalence of risk factors associated with preterm birth, such as maternal age, multiple pregnancies, and chronic health conditions, contributes to a higher demand for effective testing solutions. Companies are actively investing in research and development to introduce innovative, non-invasive, and highly sensitive diagnostic tools, aiming to improve patient outcomes and reduce the burden of premature birth on healthcare systems.
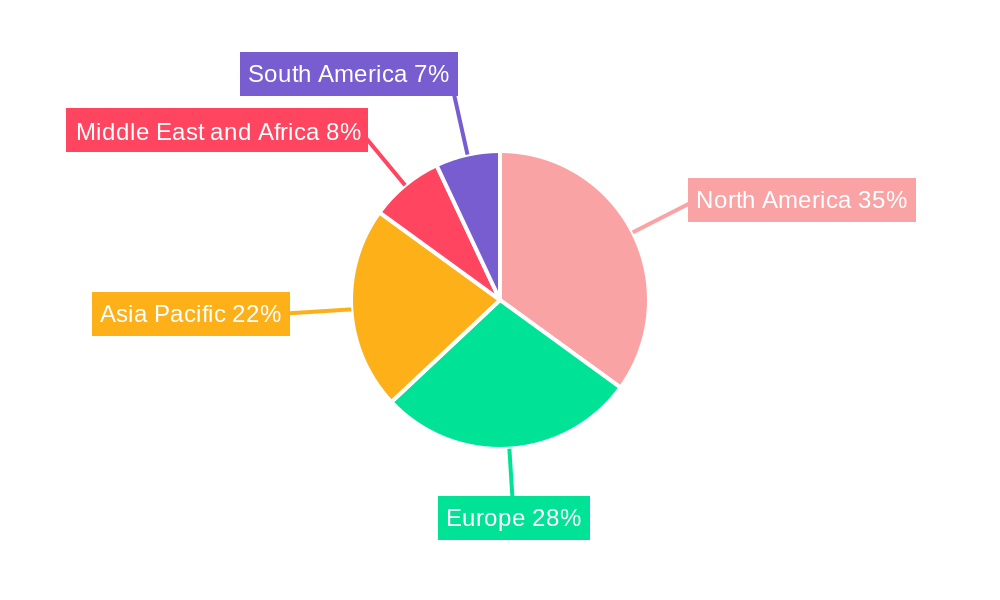
The market landscape is characterized by a diverse range of testing methodologies, with Pelvic exams, Ultrasound, and Biochemical Markers currently dominating the segment. However, the growing adoption of advanced Uterine Monitoring systems and other emerging diagnostic solutions signifies a shift towards more comprehensive and real-time monitoring of pregnancy. Geographically, North America is anticipated to lead the market share, owing to its advanced healthcare infrastructure, high adoption rates of new technologies, and substantial investment in prenatal diagnostics. Europe and Asia Pacific are also expected to witness robust growth, driven by increasing healthcare spending and a growing focus on maternal health. Key players like Sera Prognostics Inc., Hologic Inc., and Abbott are actively shaping the market through strategic collaborations, product launches, and mergers, all aimed at addressing the unmet clinical needs in preterm birth prediction and management. Despite the positive outlook, challenges such as reimbursement issues and the need for greater clinician and patient education can act as restraints, though the overarching benefits of early detection are likely to outweigh these concerns.
This in-depth report provides an exhaustive analysis of the Global Preterm Birth and PROM Testing Market, encompassing a comprehensive forecast from 2025 to 2033. With a base year of 2025, the study delves into historical trends from 2019-2024 and projects future market dynamics, revenue, and growth. We explore the critical role of early detection and intervention in improving maternal and neonatal outcomes, driven by advancements in diagnostic technologies and increasing awareness. The report targets key industry stakeholders, including manufacturers, diagnostic laboratories, healthcare providers, investors, and regulatory bodies, offering actionable insights into market opportunities and challenges.
Global Preterm Birth and PROM Testing Market Market Structure & Competitive Landscape
The Global Preterm Birth and PROM Testing Market exhibits a moderately concentrated structure, characterized by the presence of established players alongside emerging innovators. The preterm birth testing market and PROM testing market are witnessing significant innovation driven by advancements in biochemical markers and ultrasound technologies. Regulatory landscapes play a crucial role, with bodies like the FDA influencing product approvals and market access. Product substitutes, including traditional clinical assessments and less sophisticated diagnostic tools, are present but are increasingly being overshadowed by the superior accuracy and predictive power of advanced biomarker-based tests and remote uterine monitoring platforms. The end-user segmentation primarily includes hospitals, diagnostic laboratories, and specialized maternal health clinics. Mergers and acquisitions (M&A) are a notable trend, with companies aiming to consolidate market share, expand product portfolios, and enhance their R&D capabilities. For instance, the acquisition of promising early-stage diagnostic companies by larger players is anticipated to increase, further shaping the competitive arena. The overall market concentration is expected to remain moderate, fostering a dynamic competitive environment driven by technological differentiation and strategic partnerships.
Global Preterm Birth and PROM Testing Market Market Trends & Opportunities
The Global Preterm Birth and PROM Testing Market is poised for substantial growth, projected to reach an estimated value of US$XX Million by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of XX% during the forecast period (2025-2033). This surge is fueled by a confluence of factors, including a rising incidence of preterm births globally and an increasing awareness among healthcare professionals and expectant parents regarding the critical need for early identification and management of risks. Technological advancements are at the forefront of this evolution. The development and validation of novel biochemical markers for predicting preterm birth are transforming risk assessment, offering more precise and earlier insights compared to traditional methods. Furthermore, the integration of Artificial Intelligence (AI) and machine learning algorithms into diagnostic platforms is enhancing the accuracy and predictive capabilities of these tests.
Consumer preferences are also shifting towards more personalized and proactive healthcare solutions. Expectant mothers are increasingly seeking advanced diagnostic options that can provide reassurance and enable timely interventions, thereby mitigating potential complications associated with preterm birth. This demand translates into a growing market penetration for advanced preterm birth diagnostic tests and PROM diagnostic kits.
The competitive landscape is dynamic, with key players investing heavily in research and development to introduce innovative solutions. Strategic collaborations and partnerships are becoming instrumental in expanding market reach and accelerating product adoption. For example, collaborations between diagnostic companies and research institutions are crucial for validating new biomarkers and expanding the clinical utility of existing tests. The market is also witnessing a rise in point-of-care testing solutions, aiming to provide rapid and accessible diagnostic results in diverse healthcare settings.
Opportunities abound in emerging economies, where the incidence of preterm births is high, and access to advanced maternal healthcare is expanding. The development of cost-effective and accessible diagnostic solutions tailored to these markets will be a significant growth catalyst. Moreover, the increasing focus on value-based healthcare is driving the adoption of tests that demonstrate clear clinical utility and cost-effectiveness in preventing adverse maternal and neonatal outcomes. The preterm labor diagnostic market and PROM detection market are therefore integral components of the broader maternal health ecosystem, with significant potential for innovation and market expansion.
Dominant Markets & Segments in Global Preterm Birth and PROM Testing Market
The Global Preterm Birth and PROM Testing Market is segmented by Test Type, including Pelvic exam, Ultrasound, Biochemical Markers, Uterine Monitoring, and Others. Among these, Biochemical Markers are emerging as the dominant segment, driven by their increasing accuracy in predicting preterm birth and Premature Rupture of Membranes (PROM). The biochemical marker testing market is experiencing robust growth due to the development of sophisticated diagnostic panels that analyze specific proteins and biomarkers indicative of impending preterm labor or PROM.
The Ultrasound segment also holds significant market share, providing valuable insights into cervical length and fetal development, which are crucial indicators for assessing preterm birth risk. Continuous advancements in ultrasound technology, including 3D and 4D imaging, enhance diagnostic precision.
Uterine Monitoring, particularly through remote monitoring platforms like Nuvo Group's INVU, is gaining traction. The FDA approval for its expanded utility to include a uterine activity module signifies a shift towards continuous, home-based monitoring, offering proactive management of pregnancy risks. This segment is expected to witness substantial growth as remote patient monitoring becomes more integrated into standard prenatal care.
Geographically, North America currently dominates the Global Preterm Birth and PROM Testing Market, owing to high healthcare expenditure, advanced healthcare infrastructure, and a strong emphasis on prenatal care and early detection of complications. The United States, in particular, is a key market, driven by widespread adoption of innovative diagnostic technologies and supportive regulatory frameworks.
Europe follows as another significant market, characterized by well-established healthcare systems and increasing investments in maternal and child health research. Countries like Germany, the UK, and France are actively involved in the development and adoption of advanced preterm birth screening and PROM detection methods.
Emerging markets in Asia-Pacific are expected to witness the fastest growth. Factors contributing to this include a rising incidence of preterm births, increasing disposable incomes, growing awareness about maternal health, and a burgeoning healthcare sector. Government initiatives aimed at improving maternal and child health outcomes are also playing a pivotal role in driving market expansion in this region.
The "Others" segment encompasses a range of diagnostic approaches, including genetic testing and specialized clinical assessments, which, while not as prevalent as the primary segments, contribute to the overall diagnostic arsenal for preterm birth and PROM. The market dominance is thus a dynamic interplay of technological superiority, clinical utility, accessibility, and regional healthcare priorities.
Global Preterm Birth and PROM Testing Market Product Analysis
The Global Preterm Birth and PROM Testing Market is characterized by continuous product innovation, focusing on enhanced accuracy, early detection, and improved patient outcomes. Biochemical marker-based tests, such as the PreTRM test by Sera Prognostics, are revolutionizing preterm birth risk assessment by identifying specific biomarkers with high predictive value. These tests offer a significant advantage over traditional methods by providing a quantitative risk score, enabling clinicians to stratify patients and tailor management strategies proactively. Applications range from routine prenatal screening to high-risk pregnancy management. Competitive advantages lie in their non-invasive nature, high sensitivity, and specificity, leading to better clinical decision-making and potentially reducing adverse neonatal outcomes. The market also sees advancements in ultrasound technologies for more precise cervical length measurements and fetal assessments, alongside the development of innovative uterine monitoring devices for remote and continuous surveillance. These products are designed to offer early warning signs, allowing for timely intervention and improved management of high-risk pregnancies.
Key Drivers, Barriers & Challenges in Global Preterm Birth and PROM Testing Market
Key Drivers:
- Rising Incidence of Preterm Births: The global increase in preterm births necessitates advanced diagnostic tools for early detection and intervention.
- Technological Advancements: Innovations in biomarker discovery, assay development, and diagnostic imaging are enhancing the accuracy and predictive capabilities of tests.
- Increasing Maternal Health Awareness: Greater awareness among pregnant individuals and healthcare providers regarding the risks and consequences of preterm birth is driving demand for screening.
- Focus on Personalized Medicine: The shift towards personalized healthcare approaches supports the development of risk-stratified diagnostic solutions.
- Favorable Reimbursement Policies: Growing recognition of the clinical and economic benefits of early detection is leading to improved reimbursement for advanced diagnostic tests.
Barriers & Challenges:
- High Cost of Advanced Diagnostics: The initial investment and ongoing costs associated with sophisticated testing can be a barrier in resource-limited settings.
- Regulatory Hurdles: Obtaining regulatory approval for new diagnostic tests can be a lengthy and complex process, impacting time-to-market.
- Lack of Standardized Protocols: Variations in testing methodologies and interpretation can lead to inconsistencies in clinical practice.
- Physician and Patient Education: Educating healthcare providers and expectant mothers about the benefits and proper use of new diagnostic tools is crucial for adoption.
- Data Integration and Interoperability: Seamless integration of test results into existing electronic health record systems remains a challenge.
- Supply Chain Disruptions: Global supply chain vulnerabilities can impact the availability of essential reagents and equipment for diagnostic testing.
Growth Drivers in the Global Preterm Birth and PROM Testing Market Market
The Global Preterm Birth and PROM Testing Market is experiencing robust growth driven by several key factors. Technologically, the continuous discovery and validation of novel biomarkers are significantly enhancing the predictive accuracy of preterm birth and PROM tests. Advancements in assay development and molecular diagnostics are enabling the creation of more sensitive and specific tests. Economically, the increasing healthcare expenditure globally, coupled with a growing understanding of the long-term economic burden of preterm birth complications, is driving investment in early detection and prevention strategies. Regulatory bodies are also increasingly recognizing the importance of these diagnostics, which can facilitate faster market access. Furthermore, the expanding reach of telemedicine and remote patient monitoring platforms presents a significant opportunity for wider adoption of uterine monitoring technologies, contributing to proactive pregnancy management.
Challenges Impacting Global Preterm Birth and PROM Testing Market Growth
Despite promising growth prospects, the Global Preterm Birth and PROM Testing Market faces several critical challenges. Regulatory complexities, particularly in obtaining approvals for novel diagnostic technologies across different regions, can hinder market penetration. Supply chain issues, including the availability of specialized reagents and manufacturing components, can lead to production delays and impact market availability. Competitive pressures are also intensifying as new players enter the market with innovative solutions, requiring existing companies to continuously invest in R&D and marketing to maintain their market share. Furthermore, the cost-effectiveness of advanced diagnostic tests needs to be clearly demonstrated to healthcare payers and providers to ensure widespread reimbursement and adoption, especially in developing economies.
Key Players Shaping the Global Preterm Birth and PROM Testing Market Market
- Sera Prognostics Inc
- Hologic Inc
- IQ Products
- Biosynex
- Qiagen N V
- CooperSurgical Inc
- NX Prenatal Inc
- Abbott
- Clinical Innovations Inc
- LifeCell International Pvt Ltd
Significant Global Preterm Birth and PROM Testing Market Industry Milestones
- May 2022: Sera Prognostics and the Newborn Foundation launched the Every mother, every Baby Project to raise awareness about the role of validated biomarker-based preterm risk assessment tests like Sera's PreTRM test in improving maternal and newborn health.
- November 2021: Sera Prognostics announced a partnership with PreemieWorld, GLO Preemies, and the Alliance for Black NICU Families to empower and support families impacted by preterm birth.
- June 2021: Nuvo Group received United States Food and Drug Administration approval for the expanded utility of its INVU platform, adding a uterine activity module for remote monitoring of uterine activity.
Future Outlook for Global Preterm Birth and PROM Testing Market Market
- May 2022: Sera Prognostics and the Newborn Foundation launched the Every mother, every Baby Project to raise awareness about the role of validated biomarker-based preterm risk assessment tests like Sera's PreTRM test in improving maternal and newborn health.
- November 2021: Sera Prognostics announced a partnership with PreemieWorld, GLO Preemies, and the Alliance for Black NICU Families to empower and support families impacted by preterm birth.
- June 2021: Nuvo Group received United States Food and Drug Administration approval for the expanded utility of its INVU platform, adding a uterine activity module for remote monitoring of uterine activity.
Future Outlook for Global Preterm Birth and PROM Testing Market Market
The future outlook for the Global Preterm Birth and PROM Testing Market is highly promising, driven by an unwavering commitment to improving maternal and neonatal health outcomes. Strategic opportunities lie in the development of multi-marker panels that offer even greater predictive accuracy and early warning capabilities. The expansion of these diagnostic solutions into emerging markets, with tailored approaches for varying healthcare infrastructures and economic capacities, will be a key growth catalyst. Further integration of AI and machine learning into diagnostic algorithms will enhance personalized risk assessments and treatment recommendations. Continued investment in clinical validation and real-world evidence generation will solidify the value proposition of advanced preterm birth and PROM testing, driving wider adoption and ultimately contributing to a significant reduction in preterm birth rates and associated complications worldwide.
Global Preterm Birth and PROM Testing Market Segmentation
-
1. Test Type
- 1.1. Pelvic exam
- 1.2. Ultrasound
- 1.3. Biochemical Markers
- 1.4. Uterine Monitoring
- 1.5. Others
Global Preterm Birth and PROM Testing Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
Global Preterm Birth and PROM Testing Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 3.35% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Incidence of Preterm and Low-weight Births; Increasing Awareness and Technological Advancements in Point-Of Care and PROM Testing
- 3.3. Market Restrains
- 3.3.1. Lack of Awareness and Economic Constraints in Under-developed Countries
- 3.4. Market Trends
- 3.4.1. Ultrasound Segment Expects to Register a High CAGR in the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Test Type
- 5.1.1. Pelvic exam
- 5.1.2. Ultrasound
- 5.1.3. Biochemical Markers
- 5.1.4. Uterine Monitoring
- 5.1.5. Others
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.2.2. Europe
- 5.2.3. Asia Pacific
- 5.2.4. Middle East and Africa
- 5.2.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Test Type
- 6. North America Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Test Type
- 6.1.1. Pelvic exam
- 6.1.2. Ultrasound
- 6.1.3. Biochemical Markers
- 6.1.4. Uterine Monitoring
- 6.1.5. Others
- 6.1. Market Analysis, Insights and Forecast - by Test Type
- 7. Europe Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Test Type
- 7.1.1. Pelvic exam
- 7.1.2. Ultrasound
- 7.1.3. Biochemical Markers
- 7.1.4. Uterine Monitoring
- 7.1.5. Others
- 7.1. Market Analysis, Insights and Forecast - by Test Type
- 8. Asia Pacific Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Test Type
- 8.1.1. Pelvic exam
- 8.1.2. Ultrasound
- 8.1.3. Biochemical Markers
- 8.1.4. Uterine Monitoring
- 8.1.5. Others
- 8.1. Market Analysis, Insights and Forecast - by Test Type
- 9. Middle East and Africa Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Test Type
- 9.1.1. Pelvic exam
- 9.1.2. Ultrasound
- 9.1.3. Biochemical Markers
- 9.1.4. Uterine Monitoring
- 9.1.5. Others
- 9.1. Market Analysis, Insights and Forecast - by Test Type
- 10. South America Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Test Type
- 10.1.1. Pelvic exam
- 10.1.2. Ultrasound
- 10.1.3. Biochemical Markers
- 10.1.4. Uterine Monitoring
- 10.1.5. Others
- 10.1. Market Analysis, Insights and Forecast - by Test Type
- 11. North America Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 11.1.1 United States
- 11.1.2 Canada
- 11.1.3 Mexico
- 12. Europe Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1 Germany
- 12.1.2 United Kingdom
- 12.1.3 France
- 12.1.4 Italy
- 12.1.5 Spain
- 12.1.6 Rest of Europe
- 13. Asia Pacific Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1 China
- 13.1.2 Japan
- 13.1.3 India
- 13.1.4 Australia
- 13.1.5 South Korea
- 13.1.6 Rest of Asia Pacific
- 14. Middle East and Africa Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1 GCC
- 14.1.2 South Africa
- 14.1.3 Rest of Middle East and Africa
- 15. South America Global Preterm Birth and PROM Testing Market Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1 Brazil
- 15.1.2 Argentina
- 15.1.3 Rest of South America
- 16. Competitive Analysis
- 16.1. Market Share Analysis 2024
- 16.2. Company Profiles
- 16.2.1 Sera Prognostics Inc
- 16.2.1.1. Overview
- 16.2.1.2. Products
- 16.2.1.3. SWOT Analysis
- 16.2.1.4. Recent Developments
- 16.2.1.5. Financials (Based on Availability)
- 16.2.2 Hologic Inc
- 16.2.2.1. Overview
- 16.2.2.2. Products
- 16.2.2.3. SWOT Analysis
- 16.2.2.4. Recent Developments
- 16.2.2.5. Financials (Based on Availability)
- 16.2.3 IQ Products
- 16.2.3.1. Overview
- 16.2.3.2. Products
- 16.2.3.3. SWOT Analysis
- 16.2.3.4. Recent Developments
- 16.2.3.5. Financials (Based on Availability)
- 16.2.4 Biosynex
- 16.2.4.1. Overview
- 16.2.4.2. Products
- 16.2.4.3. SWOT Analysis
- 16.2.4.4. Recent Developments
- 16.2.4.5. Financials (Based on Availability)
- 16.2.5 Qiagen N V
- 16.2.5.1. Overview
- 16.2.5.2. Products
- 16.2.5.3. SWOT Analysis
- 16.2.5.4. Recent Developments
- 16.2.5.5. Financials (Based on Availability)
- 16.2.6 CooperSurgical Inc
- 16.2.6.1. Overview
- 16.2.6.2. Products
- 16.2.6.3. SWOT Analysis
- 16.2.6.4. Recent Developments
- 16.2.6.5. Financials (Based on Availability)
- 16.2.7 NX Prenatal Inc *List Not Exhaustive
- 16.2.7.1. Overview
- 16.2.7.2. Products
- 16.2.7.3. SWOT Analysis
- 16.2.7.4. Recent Developments
- 16.2.7.5. Financials (Based on Availability)
- 16.2.8 Abbott
- 16.2.8.1. Overview
- 16.2.8.2. Products
- 16.2.8.3. SWOT Analysis
- 16.2.8.4. Recent Developments
- 16.2.8.5. Financials (Based on Availability)
- 16.2.9 Clinical Innovations Inc
- 16.2.9.1. Overview
- 16.2.9.2. Products
- 16.2.9.3. SWOT Analysis
- 16.2.9.4. Recent Developments
- 16.2.9.5. Financials (Based on Availability)
- 16.2.10 LifeCell International Pvt Ltd
- 16.2.10.1. Overview
- 16.2.10.2. Products
- 16.2.10.3. SWOT Analysis
- 16.2.10.4. Recent Developments
- 16.2.10.5. Financials (Based on Availability)
- 16.2.1 Sera Prognostics Inc
List of Figures
- Figure 1: Global Global Preterm Birth and PROM Testing Market Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: North America Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 3: North America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 4: Europe Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 5: Europe Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 6: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 7: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 8: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 9: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 10: South America Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 11: South America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 12: North America Global Preterm Birth and PROM Testing Market Revenue (Million), by Test Type 2024 & 2032
- Figure 13: North America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Test Type 2024 & 2032
- Figure 14: North America Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 15: North America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 16: Europe Global Preterm Birth and PROM Testing Market Revenue (Million), by Test Type 2024 & 2032
- Figure 17: Europe Global Preterm Birth and PROM Testing Market Revenue Share (%), by Test Type 2024 & 2032
- Figure 18: Europe Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 19: Europe Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 20: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue (Million), by Test Type 2024 & 2032
- Figure 21: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue Share (%), by Test Type 2024 & 2032
- Figure 22: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 23: Asia Pacific Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 24: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue (Million), by Test Type 2024 & 2032
- Figure 25: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue Share (%), by Test Type 2024 & 2032
- Figure 26: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 27: Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
- Figure 28: South America Global Preterm Birth and PROM Testing Market Revenue (Million), by Test Type 2024 & 2032
- Figure 29: South America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Test Type 2024 & 2032
- Figure 30: South America Global Preterm Birth and PROM Testing Market Revenue (Million), by Country 2024 & 2032
- Figure 31: South America Global Preterm Birth and PROM Testing Market Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 3: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Region 2019 & 2032
- Table 4: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 5: United States Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 6: Canada Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Mexico Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 9: Germany Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: United Kingdom Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: France Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Italy Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Spain Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Rest of Europe Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 15: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 16: China Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Japan Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: India Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 19: Australia Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: South Korea Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 21: Rest of Asia Pacific Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 23: GCC Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: South Africa Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 25: Rest of Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 27: Brazil Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Argentina Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 29: Rest of South America Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 31: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 32: United States Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 33: Canada Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Mexico Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 35: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 36: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 37: Germany Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: United Kingdom Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 39: France Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 40: Italy Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 41: Spain Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 42: Rest of Europe Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 43: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 44: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 45: China Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 46: Japan Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 47: India Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: Australia Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 49: South Korea Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 50: Rest of Asia Pacific Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 51: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 52: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 53: GCC Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 54: South Africa Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 55: Rest of Middle East and Africa Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 56: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Test Type 2019 & 2032
- Table 57: Global Preterm Birth and PROM Testing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 58: Brazil Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 59: Argentina Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 60: Rest of South America Global Preterm Birth and PROM Testing Market Revenue (Million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Global Preterm Birth and PROM Testing Market?
The projected CAGR is approximately 3.35%.
2. Which companies are prominent players in the Global Preterm Birth and PROM Testing Market?
Key companies in the market include Sera Prognostics Inc, Hologic Inc, IQ Products, Biosynex, Qiagen N V, CooperSurgical Inc, NX Prenatal Inc *List Not Exhaustive, Abbott, Clinical Innovations Inc, LifeCell International Pvt Ltd.
3. What are the main segments of the Global Preterm Birth and PROM Testing Market?
The market segments include Test Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Incidence of Preterm and Low-weight Births; Increasing Awareness and Technological Advancements in Point-Of Care and PROM Testing.
6. What are the notable trends driving market growth?
Ultrasound Segment Expects to Register a High CAGR in the Forecast Period.
7. Are there any restraints impacting market growth?
Lack of Awareness and Economic Constraints in Under-developed Countries.
8. Can you provide examples of recent developments in the market?
In May 2022, Sera Prognostics and the Newborn Foundation announced the launch of the Every mother, every Baby Project. Its goal is to raise awareness among clinicians, policymakers, and public health stakeholders about the essential role validated biomarker-based preterm risk assessment tests like Sera's PreTRM test can play in improving newborn and maternal health outcomes.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Global Preterm Birth and PROM Testing Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Global Preterm Birth and PROM Testing Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Global Preterm Birth and PROM Testing Market?
To stay informed about further developments, trends, and reports in the Global Preterm Birth and PROM Testing Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


