Key Insights
The global Clinical Trial Management Systems (CTMS) market is projected to reach $21 billion by 2025, exhibiting a Compound Annual Growth Rate (CAGR) of 5.9%. This expansion is driven by increasing clinical trial complexity, the demand for efficient data management and regulatory compliance, and the acceleration of drug development. Pharmaceutical companies, Contract Research Organizations (CROs), and healthcare entities are adopting CTMS to optimize trial operations, improve data accuracy, reduce costs, and expedite therapy launches. The trend towards scalable and accessible cloud-based solutions is reshaping the market, alongside crucial services like implementation and support.
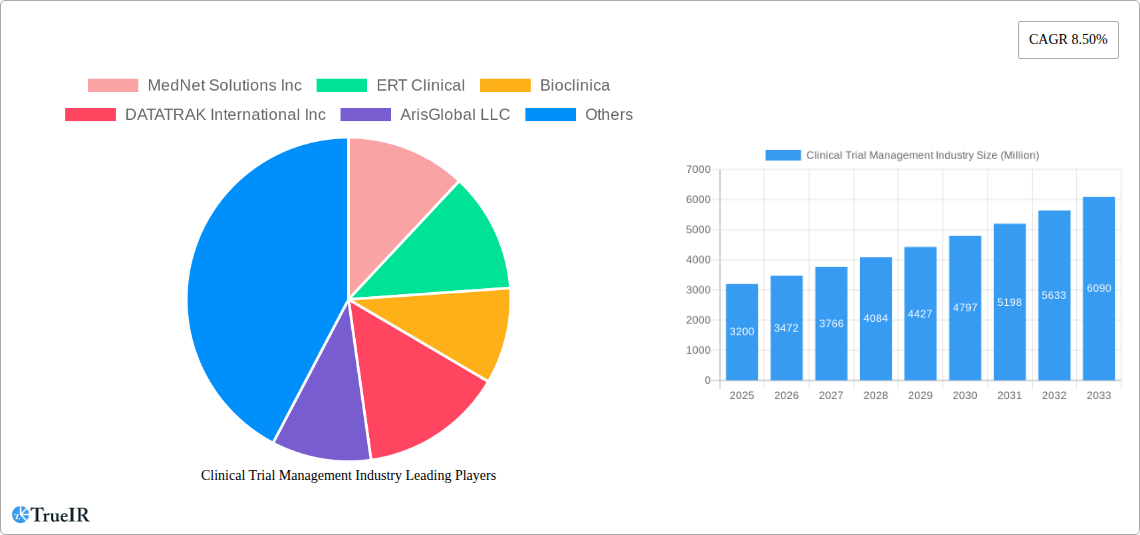
Clinical Trial Management Industry Market Size (In Billion)

Key growth drivers include the expanding global pharmaceutical sector, rising healthcare expenditures, and stringent regulatory mandates requiring robust data integrity and reporting systems. Emerging markets, particularly in the Asia Pacific, are experiencing significant growth due to healthcare infrastructure investments and increased research activities. While high initial investment costs and integration challenges exist, the benefits of enhanced operational efficiency, patient safety, and accelerated drug approvals are expected to drive sustained market growth.
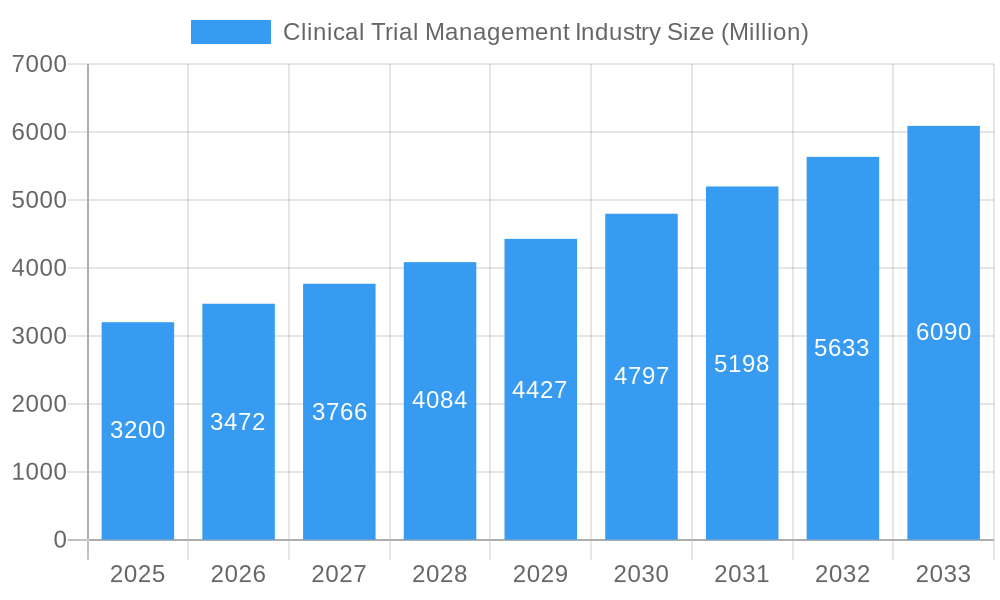
Clinical Trial Management Industry Company Market Share

This report offers a comprehensive analysis of the global Clinical Trial Management (CTM) market, providing critical insights for stakeholders. Covering the historical period of 2019–2024 and projecting growth through 2033, with a base year of 2025, this research examines market structure, trends, opportunities, dominant segments, product innovations, key players, and future outlook.
Clinical Trial Management Industry Market Structure & Competitive Landscape
The Clinical Trial Management (CTM) industry exhibits a moderately concentrated market structure, with a handful of leading players holding significant market share. Innovation remains a key driver, propelled by the relentless pursuit of enhanced efficiency, data integrity, and patient centricity in clinical research. Regulatory impacts, particularly from agencies like the FDA and EMA, exert considerable influence, shaping operational standards and demanding robust compliance solutions. Product substitutes, while present in the form of manual processes or disparate systems, are increasingly being phased out in favor of integrated CTM solutions. End-user segmentation reveals a dominant focus on Pharmaceuticals and Contract Research Organizations (CROs), followed by other research entities. Mergers and Acquisitions (M&A) activity is a notable trend, indicating consolidation and strategic expansion as companies seek to broaden their service offerings and geographical reach. For instance, the past five years have seen an average of XX M&A deals annually, with deal values ranging from xx Million to xx Million. Concentration ratios for the top 3 players stand at approximately XX%, highlighting the competitive intensity.
Clinical Trial Management Industry Market Trends & Opportunities
The global Clinical Trial Management (CTM) market is poised for substantial growth, projected to reach a valuation of over $XX Million by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of XX% from 2025 to 2033. This expansion is fueled by several intersecting trends. The increasing complexity and global nature of clinical trials necessitate sophisticated management solutions to ensure seamless execution and data accuracy. A significant technological shift is the pervasive adoption of cloud-based CTM solutions, offering enhanced scalability, accessibility, and cost-effectiveness compared to on-premise alternatives. This shift is reflected in cloud-based solutions capturing approximately XX% of the market share in 2025.
Consumer preferences, specifically the growing demand for decentralized clinical trials (DCTs) and patient-centric approaches, are driving the development of CTM platforms that facilitate remote monitoring, telemedicine integration, and patient engagement. The rising prevalence of chronic diseases and the subsequent increase in research and development activities, particularly in oncology and rare diseases, further stimulate demand for efficient CTM systems. Competitive dynamics are intensifying, with established players investing heavily in R&D to integrate artificial intelligence (AI), machine learning (ML), and blockchain technologies for predictive analytics, fraud detection, and enhanced data security. The market penetration rate for advanced CTM software solutions is projected to increase from XX% in 2024 to XX% by 2033. Opportunities abound for CTM providers offering integrated solutions encompassing electronic data capture (EDC), electronic trial master file (eTMF), clinical trial budgeting and payments, and site management functionalities. Furthermore, the growing emphasis on real-world evidence (RWE) collection is opening new avenues for CTM platforms capable of integrating diverse data sources. The evolving regulatory landscape, with a focus on data standardization and interoperability, also presents an opportunity for CTM vendors to offer compliant and future-proof solutions.
Dominant Markets & Segments in Clinical Trial Management Industry
The Cloud-based delivery mode is demonstrably dominant within the Clinical Trial Management (CTM) industry, capturing an estimated XX% market share in 2025. This segment is experiencing robust growth driven by its inherent flexibility, scalability, and reduced IT infrastructure burden for pharmaceutical companies and contract research organizations. Key growth drivers for cloud-based solutions include the increasing need for remote access to trial data, enhanced collaboration among global research teams, and the ability to rapidly deploy and update software.
Within the Component segmentation, Software solutions hold the largest market share, projected at XX% in 2025. This is further bifurcated into various modules such as electronic data capture (EDC), electronic trial master file (eTMF), clinical trial management systems (CTMS), and clinical trial budgeting and payment solutions. The increasing digitization of clinical trial processes and the demand for comprehensive data management and regulatory compliance are propelling the software segment's growth.
From an End User perspective, the Pharmaceuticals sector is the leading market, accounting for an estimated XX% of the CTM market in 2025. The pharmaceutical industry's extensive investment in drug development and the high number of clinical trials conducted annually make them the primary consumers of CTM solutions. Contract Research Organizations (CROs) represent the second-largest end-user segment, holding approximately XX% of the market share, as they increasingly leverage CTM platforms to manage trials on behalf of their pharmaceutical clients.
Geographically, North America is expected to remain the dominant region, driven by the presence of a large number of pharmaceutical companies, robust research infrastructure, and favorable regulatory environments. Within North America, the United States is the leading country, contributing an estimated XX% to the global CTM market revenue. Advanced healthcare systems, significant R&D spending, and a well-established network of clinical trial sites contribute to this dominance. Emerging markets in Asia-Pacific are also showing significant growth potential due to increasing healthcare investments and a growing number of clinical trials being conducted.
Clinical Trial Management Industry Product Analysis
Product innovation in the Clinical Trial Management (CTM) industry is characterized by the integration of advanced technologies to streamline trial execution and enhance data intelligence. Solutions are increasingly incorporating AI and machine learning for predictive analytics, risk-based monitoring, and protocol optimization. Applications span from enhanced patient recruitment and retention tools to sophisticated eTMF management and real-time data visualization dashboards. Competitive advantages lie in platforms offering seamless integration across various trial components, robust security features, and user-friendly interfaces that cater to the evolving needs of pharmaceutical companies, CROs, and research sites.
Key Drivers, Barriers & Challenges in Clinical Trial Management Industry
Key Drivers:
- Increasing R&D Investments: Growing expenditure in drug discovery and development fuels demand for efficient CTM solutions.
- Technological Advancements: AI, ML, and cloud computing are enhancing CTM capabilities.
- Regulatory Compliance Demands: Stringent regulations necessitate robust data management and reporting tools.
- Rise of Decentralized Clinical Trials (DCTs): Demand for remote monitoring and patient-centric solutions.
- Globalization of Clinical Trials: Need for integrated platforms to manage multi-site, multi-country studies.
Barriers & Challenges:
- High Implementation Costs: Initial investment in CTM software and infrastructure can be substantial.
- Data Security and Privacy Concerns: Protecting sensitive patient data remains a critical challenge.
- Integration with Legacy Systems: Difficulty in integrating new CTM solutions with existing IT infrastructure.
- Regulatory Hurdles in Emerging Markets: Navigating diverse and evolving regulatory landscapes.
- Skilled Workforce Shortage: Demand for trained professionals to manage and operate CTM systems. The estimated impact of these challenges on market growth is a reduction of approximately XX% in potential revenue.
Growth Drivers in the Clinical Trial Management Industry Market
The Clinical Trial Management (CTM) industry is experiencing significant growth driven by a confluence of factors. Technological innovation is a primary catalyst, with the integration of artificial intelligence (AI) and machine learning (ML) into CTM platforms enabling predictive analytics, automated data review, and enhanced operational efficiency. The increasing complexity and global nature of clinical trials, coupled with a rise in chronic disease prevalence, are augmenting the need for sophisticated, integrated CTM solutions. Furthermore, favorable government initiatives and funding for medical research, alongside a growing emphasis on patient-centric trial designs and decentralized clinical trials (DCTs), are creating substantial market opportunities.
Challenges Impacting Clinical Trial Management Industry Growth
Despite the robust growth trajectory, the Clinical Trial Management (CTM) industry faces several significant challenges. High implementation costs and the complexity of integrating new CTM systems with existing legacy infrastructure can pose substantial barriers for organizations, particularly smaller ones. Data security and patient privacy concerns are paramount, demanding sophisticated cybersecurity measures and compliance with evolving data protection regulations. Navigating the diverse and often stringent regulatory landscapes across different countries adds another layer of complexity. Furthermore, a persistent shortage of skilled professionals trained in CTM software and clinical trial operations can hinder adoption and efficient utilization of these technologies, impacting market expansion by an estimated XX%.
Key Players Shaping the Clinical Trial Management Industry Market
- MedNet Solutions Inc
- ERT Clinical
- Bioclinica
- DATATRAK International Inc
- ArisGlobal LLC
- RealTime Software Solutions LLC
- Advarra
- DZS Clinical Services
- Oracle Corporation
- Veeva Systems
- Calyx
- Dassault Systèmes (Medidata Solutions Inc)
- IBM
Significant Clinical Trial Management Industry Industry Milestones
- March 2023: Assentia launched tech platforms to support payments in the clinical trial space, releasing two SaaS-based applications, GrantPay and GrantPact, to provide clinical trial contract negotiation and payment services.
- February 2023: Vial partnered with Egnyte. By integrating Egnyte's eTMF, Vial will be able to offer clients the gold standard in eTMF management, compliance, and audit readiness with the increased bar in clinical trial technology.
Future Outlook for Clinical Trial Management Industry Market
The future outlook for the Clinical Trial Management (CTM) industry is exceptionally promising, driven by continued technological innovation and the increasing demand for efficient, compliant, and patient-centric clinical research. Strategic opportunities lie in the expansion of AI and ML capabilities for advanced data analytics, predictive modeling, and automated operational tasks. The growing adoption of decentralized clinical trials (DCTs) will further spur innovation in remote monitoring and patient engagement solutions. Furthermore, the increasing focus on real-world data (RWD) and real-world evidence (RWE) presents a significant avenue for CTM platforms to integrate and analyze diverse datasets. The market is expected to witness continued consolidation and strategic partnerships as companies strive to offer comprehensive, end-to-end CTM solutions, ultimately accelerating drug development timelines and improving patient outcomes.
Clinical Trial Management Industry Segmentation
-
1. Delivery Mode
- 1.1. On-premise
- 1.2. Cloud-based
-
2. Component
- 2.1. Software
- 2.2. Services
-
3. End User
- 3.1. Pharmaceuticals
- 3.2. Contract Research Organization
- 3.3. Other End Users
Clinical Trial Management Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
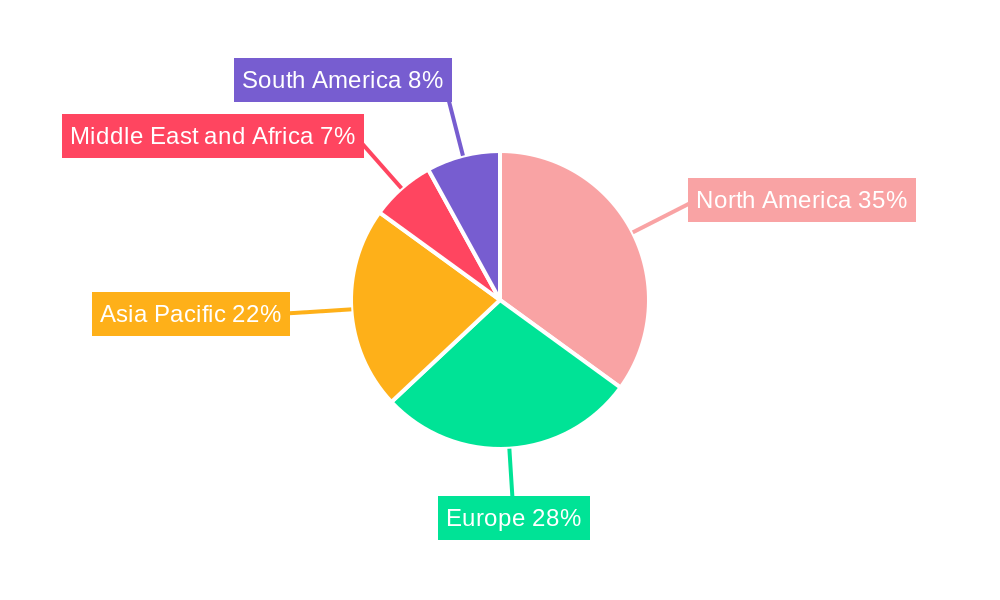
Clinical Trial Management Industry Regional Market Share

Geographic Coverage of Clinical Trial Management Industry
Clinical Trial Management Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.9% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Growing Number of Clinical Trials Due to Rising Chronic Diseases and Lifestyle-related Disorders; Rise in Outsourcing of Clinical Trials and Implementation by Contract Research Organizations
- 3.3. Market Restrains
- 3.3.1. Data Security Issues; High Cost Associated With Clinical Trial Management Systems
- 3.4. Market Trends
- 3.4.1. The Pharmaceutical Segment is Expected to Grow Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 5.1.1. On-premise
- 5.1.2. Cloud-based
- 5.2. Market Analysis, Insights and Forecast - by Component
- 5.2.1. Software
- 5.2.2. Services
- 5.3. Market Analysis, Insights and Forecast - by End User
- 5.3.1. Pharmaceuticals
- 5.3.2. Contract Research Organization
- 5.3.3. Other End Users
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 6. North America Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 6.1.1. On-premise
- 6.1.2. Cloud-based
- 6.2. Market Analysis, Insights and Forecast - by Component
- 6.2.1. Software
- 6.2.2. Services
- 6.3. Market Analysis, Insights and Forecast - by End User
- 6.3.1. Pharmaceuticals
- 6.3.2. Contract Research Organization
- 6.3.3. Other End Users
- 6.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 7. Europe Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 7.1.1. On-premise
- 7.1.2. Cloud-based
- 7.2. Market Analysis, Insights and Forecast - by Component
- 7.2.1. Software
- 7.2.2. Services
- 7.3. Market Analysis, Insights and Forecast - by End User
- 7.3.1. Pharmaceuticals
- 7.3.2. Contract Research Organization
- 7.3.3. Other End Users
- 7.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 8. Asia Pacific Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 8.1.1. On-premise
- 8.1.2. Cloud-based
- 8.2. Market Analysis, Insights and Forecast - by Component
- 8.2.1. Software
- 8.2.2. Services
- 8.3. Market Analysis, Insights and Forecast - by End User
- 8.3.1. Pharmaceuticals
- 8.3.2. Contract Research Organization
- 8.3.3. Other End Users
- 8.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 9. Middle East and Africa Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 9.1.1. On-premise
- 9.1.2. Cloud-based
- 9.2. Market Analysis, Insights and Forecast - by Component
- 9.2.1. Software
- 9.2.2. Services
- 9.3. Market Analysis, Insights and Forecast - by End User
- 9.3.1. Pharmaceuticals
- 9.3.2. Contract Research Organization
- 9.3.3. Other End Users
- 9.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 10. South America Clinical Trial Management Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 10.1.1. On-premise
- 10.1.2. Cloud-based
- 10.2. Market Analysis, Insights and Forecast - by Component
- 10.2.1. Software
- 10.2.2. Services
- 10.3. Market Analysis, Insights and Forecast - by End User
- 10.3.1. Pharmaceuticals
- 10.3.2. Contract Research Organization
- 10.3.3. Other End Users
- 10.1. Market Analysis, Insights and Forecast - by Delivery Mode
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 MedNet Solutions Inc
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 ERT Clinical
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Bioclinica
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 DATATRAK International Inc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 ArisGlobal LLC
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 RealTime Software Solutions LLC
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Advarra
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 DZS Clinical Services
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Oracle Corporation
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Veeva Systems
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Calyx
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Dassault Systèmes (Medidata Solutions Inc )
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 IBM
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.1 MedNet Solutions Inc
List of Figures
- Figure 1: Global Clinical Trial Management Industry Revenue Breakdown (billion, %) by Region 2025 & 2033
- Figure 2: Global Clinical Trial Management Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Clinical Trial Management Industry Revenue (billion), by Delivery Mode 2025 & 2033
- Figure 4: North America Clinical Trial Management Industry Volume (K Unit), by Delivery Mode 2025 & 2033
- Figure 5: North America Clinical Trial Management Industry Revenue Share (%), by Delivery Mode 2025 & 2033
- Figure 6: North America Clinical Trial Management Industry Volume Share (%), by Delivery Mode 2025 & 2033
- Figure 7: North America Clinical Trial Management Industry Revenue (billion), by Component 2025 & 2033
- Figure 8: North America Clinical Trial Management Industry Volume (K Unit), by Component 2025 & 2033
- Figure 9: North America Clinical Trial Management Industry Revenue Share (%), by Component 2025 & 2033
- Figure 10: North America Clinical Trial Management Industry Volume Share (%), by Component 2025 & 2033
- Figure 11: North America Clinical Trial Management Industry Revenue (billion), by End User 2025 & 2033
- Figure 12: North America Clinical Trial Management Industry Volume (K Unit), by End User 2025 & 2033
- Figure 13: North America Clinical Trial Management Industry Revenue Share (%), by End User 2025 & 2033
- Figure 14: North America Clinical Trial Management Industry Volume Share (%), by End User 2025 & 2033
- Figure 15: North America Clinical Trial Management Industry Revenue (billion), by Country 2025 & 2033
- Figure 16: North America Clinical Trial Management Industry Volume (K Unit), by Country 2025 & 2033
- Figure 17: North America Clinical Trial Management Industry Revenue Share (%), by Country 2025 & 2033
- Figure 18: North America Clinical Trial Management Industry Volume Share (%), by Country 2025 & 2033
- Figure 19: Europe Clinical Trial Management Industry Revenue (billion), by Delivery Mode 2025 & 2033
- Figure 20: Europe Clinical Trial Management Industry Volume (K Unit), by Delivery Mode 2025 & 2033
- Figure 21: Europe Clinical Trial Management Industry Revenue Share (%), by Delivery Mode 2025 & 2033
- Figure 22: Europe Clinical Trial Management Industry Volume Share (%), by Delivery Mode 2025 & 2033
- Figure 23: Europe Clinical Trial Management Industry Revenue (billion), by Component 2025 & 2033
- Figure 24: Europe Clinical Trial Management Industry Volume (K Unit), by Component 2025 & 2033
- Figure 25: Europe Clinical Trial Management Industry Revenue Share (%), by Component 2025 & 2033
- Figure 26: Europe Clinical Trial Management Industry Volume Share (%), by Component 2025 & 2033
- Figure 27: Europe Clinical Trial Management Industry Revenue (billion), by End User 2025 & 2033
- Figure 28: Europe Clinical Trial Management Industry Volume (K Unit), by End User 2025 & 2033
- Figure 29: Europe Clinical Trial Management Industry Revenue Share (%), by End User 2025 & 2033
- Figure 30: Europe Clinical Trial Management Industry Volume Share (%), by End User 2025 & 2033
- Figure 31: Europe Clinical Trial Management Industry Revenue (billion), by Country 2025 & 2033
- Figure 32: Europe Clinical Trial Management Industry Volume (K Unit), by Country 2025 & 2033
- Figure 33: Europe Clinical Trial Management Industry Revenue Share (%), by Country 2025 & 2033
- Figure 34: Europe Clinical Trial Management Industry Volume Share (%), by Country 2025 & 2033
- Figure 35: Asia Pacific Clinical Trial Management Industry Revenue (billion), by Delivery Mode 2025 & 2033
- Figure 36: Asia Pacific Clinical Trial Management Industry Volume (K Unit), by Delivery Mode 2025 & 2033
- Figure 37: Asia Pacific Clinical Trial Management Industry Revenue Share (%), by Delivery Mode 2025 & 2033
- Figure 38: Asia Pacific Clinical Trial Management Industry Volume Share (%), by Delivery Mode 2025 & 2033
- Figure 39: Asia Pacific Clinical Trial Management Industry Revenue (billion), by Component 2025 & 2033
- Figure 40: Asia Pacific Clinical Trial Management Industry Volume (K Unit), by Component 2025 & 2033
- Figure 41: Asia Pacific Clinical Trial Management Industry Revenue Share (%), by Component 2025 & 2033
- Figure 42: Asia Pacific Clinical Trial Management Industry Volume Share (%), by Component 2025 & 2033
- Figure 43: Asia Pacific Clinical Trial Management Industry Revenue (billion), by End User 2025 & 2033
- Figure 44: Asia Pacific Clinical Trial Management Industry Volume (K Unit), by End User 2025 & 2033
- Figure 45: Asia Pacific Clinical Trial Management Industry Revenue Share (%), by End User 2025 & 2033
- Figure 46: Asia Pacific Clinical Trial Management Industry Volume Share (%), by End User 2025 & 2033
- Figure 47: Asia Pacific Clinical Trial Management Industry Revenue (billion), by Country 2025 & 2033
- Figure 48: Asia Pacific Clinical Trial Management Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Asia Pacific Clinical Trial Management Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Asia Pacific Clinical Trial Management Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: Middle East and Africa Clinical Trial Management Industry Revenue (billion), by Delivery Mode 2025 & 2033
- Figure 52: Middle East and Africa Clinical Trial Management Industry Volume (K Unit), by Delivery Mode 2025 & 2033
- Figure 53: Middle East and Africa Clinical Trial Management Industry Revenue Share (%), by Delivery Mode 2025 & 2033
- Figure 54: Middle East and Africa Clinical Trial Management Industry Volume Share (%), by Delivery Mode 2025 & 2033
- Figure 55: Middle East and Africa Clinical Trial Management Industry Revenue (billion), by Component 2025 & 2033
- Figure 56: Middle East and Africa Clinical Trial Management Industry Volume (K Unit), by Component 2025 & 2033
- Figure 57: Middle East and Africa Clinical Trial Management Industry Revenue Share (%), by Component 2025 & 2033
- Figure 58: Middle East and Africa Clinical Trial Management Industry Volume Share (%), by Component 2025 & 2033
- Figure 59: Middle East and Africa Clinical Trial Management Industry Revenue (billion), by End User 2025 & 2033
- Figure 60: Middle East and Africa Clinical Trial Management Industry Volume (K Unit), by End User 2025 & 2033
- Figure 61: Middle East and Africa Clinical Trial Management Industry Revenue Share (%), by End User 2025 & 2033
- Figure 62: Middle East and Africa Clinical Trial Management Industry Volume Share (%), by End User 2025 & 2033
- Figure 63: Middle East and Africa Clinical Trial Management Industry Revenue (billion), by Country 2025 & 2033
- Figure 64: Middle East and Africa Clinical Trial Management Industry Volume (K Unit), by Country 2025 & 2033
- Figure 65: Middle East and Africa Clinical Trial Management Industry Revenue Share (%), by Country 2025 & 2033
- Figure 66: Middle East and Africa Clinical Trial Management Industry Volume Share (%), by Country 2025 & 2033
- Figure 67: South America Clinical Trial Management Industry Revenue (billion), by Delivery Mode 2025 & 2033
- Figure 68: South America Clinical Trial Management Industry Volume (K Unit), by Delivery Mode 2025 & 2033
- Figure 69: South America Clinical Trial Management Industry Revenue Share (%), by Delivery Mode 2025 & 2033
- Figure 70: South America Clinical Trial Management Industry Volume Share (%), by Delivery Mode 2025 & 2033
- Figure 71: South America Clinical Trial Management Industry Revenue (billion), by Component 2025 & 2033
- Figure 72: South America Clinical Trial Management Industry Volume (K Unit), by Component 2025 & 2033
- Figure 73: South America Clinical Trial Management Industry Revenue Share (%), by Component 2025 & 2033
- Figure 74: South America Clinical Trial Management Industry Volume Share (%), by Component 2025 & 2033
- Figure 75: South America Clinical Trial Management Industry Revenue (billion), by End User 2025 & 2033
- Figure 76: South America Clinical Trial Management Industry Volume (K Unit), by End User 2025 & 2033
- Figure 77: South America Clinical Trial Management Industry Revenue Share (%), by End User 2025 & 2033
- Figure 78: South America Clinical Trial Management Industry Volume Share (%), by End User 2025 & 2033
- Figure 79: South America Clinical Trial Management Industry Revenue (billion), by Country 2025 & 2033
- Figure 80: South America Clinical Trial Management Industry Volume (K Unit), by Country 2025 & 2033
- Figure 81: South America Clinical Trial Management Industry Revenue Share (%), by Country 2025 & 2033
- Figure 82: South America Clinical Trial Management Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 2: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 3: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 4: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 5: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 6: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 7: Global Clinical Trial Management Industry Revenue billion Forecast, by Region 2020 & 2033
- Table 8: Global Clinical Trial Management Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 9: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 10: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 11: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 12: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 13: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 14: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 15: Global Clinical Trial Management Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 16: Global Clinical Trial Management Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 17: United States Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 18: United States Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Canada Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 20: Canada Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 21: Mexico Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 22: Mexico Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 23: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 24: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 25: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 26: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 27: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 28: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 29: Global Clinical Trial Management Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 30: Global Clinical Trial Management Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 31: Germany Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 32: Germany Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: United Kingdom Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 34: United Kingdom Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: France Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 36: France Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Italy Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 38: Italy Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 39: Spain Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 40: Spain Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 41: Rest of Europe Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 42: Rest of Europe Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 43: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 44: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 45: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 46: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 47: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 48: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 49: Global Clinical Trial Management Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 50: Global Clinical Trial Management Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 51: China Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 52: China Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Japan Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 54: Japan Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: India Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 56: India Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 57: Australia Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 58: Australia Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 59: South Korea Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 60: South Korea Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 61: Rest of Asia Pacific Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 62: Rest of Asia Pacific Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 64: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 65: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 66: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 67: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 68: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 69: Global Clinical Trial Management Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 70: Global Clinical Trial Management Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 71: GCC Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 72: GCC Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 73: South Africa Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 74: South Africa Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Rest of Middle East and Africa Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 76: Rest of Middle East and Africa Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Global Clinical Trial Management Industry Revenue billion Forecast, by Delivery Mode 2020 & 2033
- Table 78: Global Clinical Trial Management Industry Volume K Unit Forecast, by Delivery Mode 2020 & 2033
- Table 79: Global Clinical Trial Management Industry Revenue billion Forecast, by Component 2020 & 2033
- Table 80: Global Clinical Trial Management Industry Volume K Unit Forecast, by Component 2020 & 2033
- Table 81: Global Clinical Trial Management Industry Revenue billion Forecast, by End User 2020 & 2033
- Table 82: Global Clinical Trial Management Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 83: Global Clinical Trial Management Industry Revenue billion Forecast, by Country 2020 & 2033
- Table 84: Global Clinical Trial Management Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 85: Brazil Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 86: Brazil Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 87: Argentina Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 88: Argentina Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 89: Rest of South America Clinical Trial Management Industry Revenue (billion) Forecast, by Application 2020 & 2033
- Table 90: Rest of South America Clinical Trial Management Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Clinical Trial Management Industry?
The projected CAGR is approximately 5.9%.
2. Which companies are prominent players in the Clinical Trial Management Industry?
Key companies in the market include MedNet Solutions Inc, ERT Clinical, Bioclinica, DATATRAK International Inc, ArisGlobal LLC, RealTime Software Solutions LLC, Advarra, DZS Clinical Services, Oracle Corporation, Veeva Systems, Calyx, Dassault Systèmes (Medidata Solutions Inc ), IBM.
3. What are the main segments of the Clinical Trial Management Industry?
The market segments include Delivery Mode, Component, End User.
4. Can you provide details about the market size?
The market size is estimated to be USD 21 billion as of 2022.
5. What are some drivers contributing to market growth?
Growing Number of Clinical Trials Due to Rising Chronic Diseases and Lifestyle-related Disorders; Rise in Outsourcing of Clinical Trials and Implementation by Contract Research Organizations.
6. What are the notable trends driving market growth?
The Pharmaceutical Segment is Expected to Grow Over the Forecast Period.
7. Are there any restraints impacting market growth?
Data Security Issues; High Cost Associated With Clinical Trial Management Systems.
8. Can you provide examples of recent developments in the market?
March 2023: Assentia launched tech platforms to support payments in the clinical trial space. The company released two SaaS-based applications, GrantPay and GrantPact, to provide clinical trial contract negotiation and payment services.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Clinical Trial Management Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Clinical Trial Management Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Clinical Trial Management Industry?
To stay informed about further developments, trends, and reports in the Clinical Trial Management Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
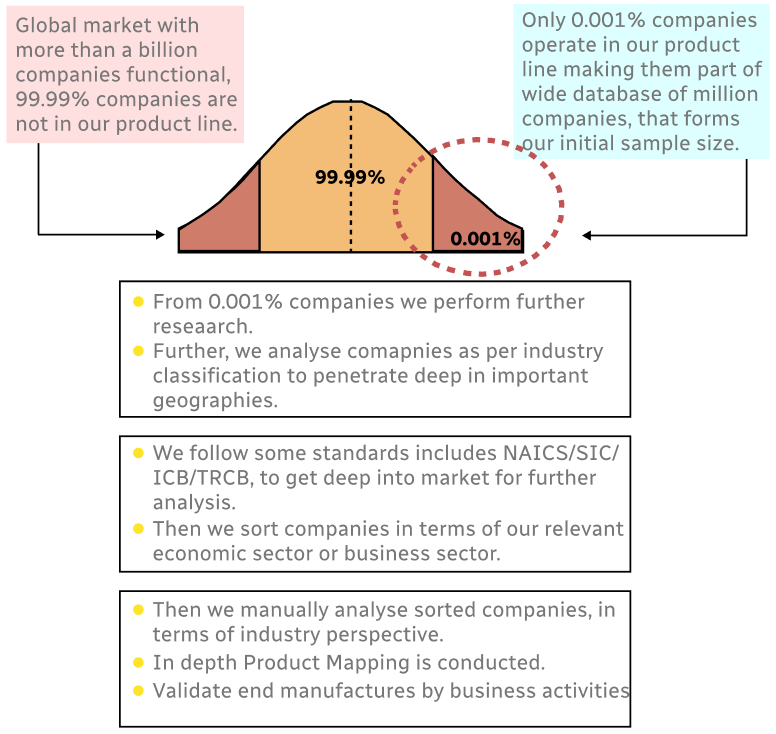
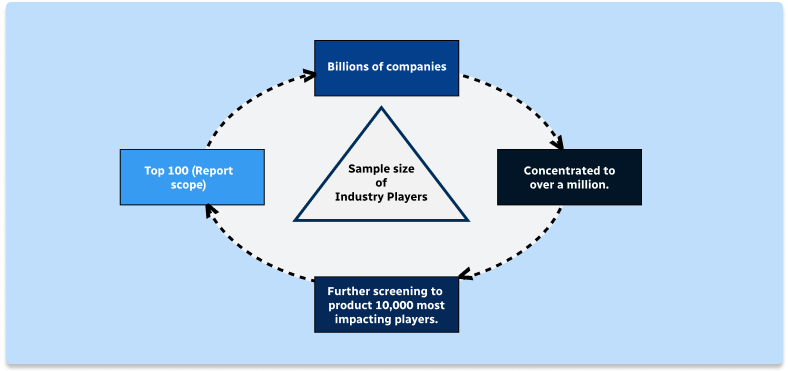
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
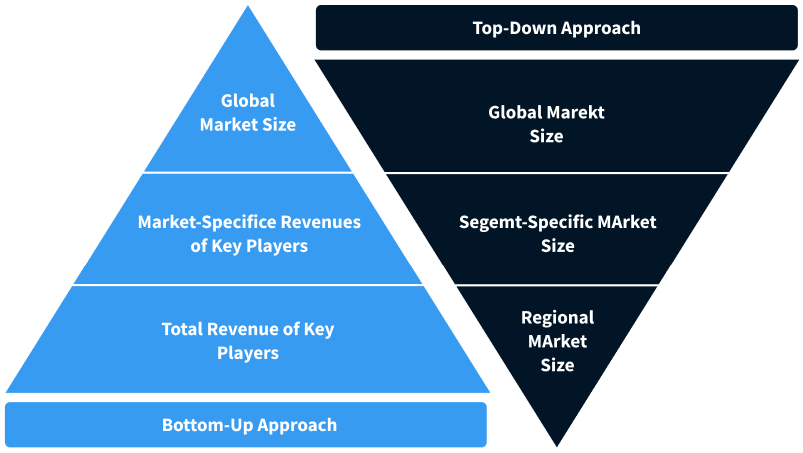
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
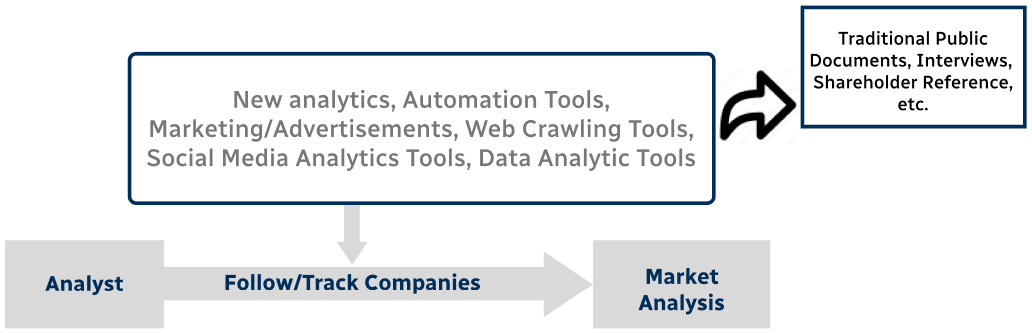
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


