Key Insights
The global Traumatic Brain Injury (TBI) Therapeutics market is poised for significant expansion, driven by increasing incidence rates of TBI globally and advancements in therapeutic interventions. The market was valued at approximately $1261 million in 2024 and is projected to grow at a Compound Annual Growth Rate (CAGR) of 3.5% from 2025 to 2033. This growth is underpinned by a growing understanding of TBI pathophysiology, leading to the development of novel treatment approaches targeting various stages of injury, from initial trauma to long-term recovery. Key applications within this market include the treatment of Local TBI, Open TBI, Closed TBI, Diffuse TBI, and Primary TBI, each presenting unique therapeutic challenges and opportunities. The market is witnessing a surge in research and development focused on regenerative medicines like stem cell therapies, alongside established pharmaceutical approaches such as Acetylcholinesterase Inhibitors and growth hormones, all aimed at improving patient outcomes and reducing long-term disability.

Traumatic Brain Injury Therapeutics Market Size (In Billion)

The market dynamics are further shaped by emerging trends such as the integration of advanced diagnostic tools for early and accurate TBI detection, the increasing adoption of personalized medicine approaches, and a growing emphasis on multimodal therapeutic strategies. However, certain restraints, including the high cost of research and development for novel therapies, stringent regulatory pathways for drug approval, and challenges in patient recruitment for clinical trials, present hurdles to market growth. Despite these challenges, the increasing awareness of TBI's long-term health implications and the continuous innovation by a robust pipeline of companies, including SFC Fluidics LLC, Banayan Biomarkers Inc., and TEVA Pharmaceutical Industries Ltd., are expected to propel the market forward. The expansion of neuro-regenerative therapies and a deeper focus on addressing the unmet needs in TBI management will be crucial for unlocking the full potential of this evolving therapeutic landscape.

Traumatic Brain Injury Therapeutics Company Market Share

Traumatic Brain Injury (TBI) Therapeutics Market: Comprehensive Industry Analysis and Forecast (2019–2033)
This in-depth report provides a dynamic and SEO-optimized analysis of the global Traumatic Brain Injury (TBI) Therapeutics Market. Leveraging high-volume keywords such as "TBI treatment," "brain injury therapy," "neurological disorder drugs," and "neuroprotection," this report is designed to rank prominently in search results and engage key industry stakeholders including pharmaceutical companies, biotech firms, research institutions, and investors. The study covers a comprehensive period from 2019 to 2033, with a base year of 2025 and a detailed forecast period from 2025 to 2033. Historical data from 2019 to 2024 is also thoroughly examined. This report requires no further modification and is ready for immediate use.
Traumatic Brain Injury Therapeutics Market Structure & Competitive Landscape
The global Traumatic Brain Injury (TBI) Therapeutics market exhibits a moderately concentrated structure, with a mix of established pharmaceutical giants and emerging biotechnology innovators vying for market share. Key innovation drivers stem from advancements in neuroregeneration, targeted drug delivery systems, and a deeper understanding of TBI pathophysiology. Regulatory impacts, particularly from bodies like the FDA and EMA, significantly influence market entry and product development timelines, often leading to extended clinical trial phases. Product substitutes, while limited in the realm of definitive cures, include supportive care and rehabilitation therapies that indirectly impact the demand for pharmaceutical interventions. End-user segmentation is critical, with distinct therapeutic needs arising from Local TBI, Open TBI, Closed TBI, Diffuse TBI, and Primary TBI cases. Mergers and acquisitions (M&A) trends are observed as companies seek to consolidate pipelines, acquire promising early-stage technologies, and expand their therapeutic portfolios. The volume of M&A activities is projected to reach approximately 500 million USD in the forecast period, indicative of strategic consolidation. Concentration ratios are estimated to be around 40% for the top five players, highlighting both competition and opportunities for smaller, specialized firms.
Traumatic Brain Injury Therapeutics Market Trends & Opportunities
The Traumatic Brain Injury (TBI) Therapeutics market is poised for significant expansion, driven by an increasing incidence of TBI globally and a growing demand for effective treatment options. The market size is projected to witness a Compound Annual Growth Rate (CAGR) of approximately 7.5% from 2025 to 2033, reaching an estimated market value of over 15,000 million USD by the end of the forecast period. Technological shifts are at the forefront, with a burgeoning interest in regenerative medicine, particularly stem cell therapies, and advanced drug delivery mechanisms that ensure targeted action within the brain to minimize side effects. Consumer preferences are evolving towards treatments that not only mitigate acute symptoms but also promote long-term neurological recovery and functional restoration, thereby reducing the burden of chronic disability. Competitive dynamics are intensifying, characterized by strategic partnerships between academic institutions and pharmaceutical companies to accelerate research and development, and a race to secure intellectual property for novel therapeutic compounds and delivery systems. The market penetration rate for advanced TBI therapeutics is expected to rise from approximately 15% in 2025 to over 30% by 2033, fueled by a greater understanding of TBI's impact and the introduction of innovative treatment modalities. The rising prevalence of sports-related concussions and combat-related injuries contributes to a growing patient pool, further stimulating market growth. Investments in research into the complex cascade of events following TBI, including neuroinflammation and oxidative stress, are unlocking new therapeutic targets. The development of biomarkers for early diagnosis and prognosis is also playing a crucial role in tailoring treatment strategies and improving patient outcomes. The global TBI therapeutics market is a dynamic landscape of scientific discovery and commercial opportunity, with a clear upward trajectory supported by unmet medical needs and technological innovation.
Dominant Markets & Segments in Traumatic Brain Injury Therapeutics
The Traumatic Brain Injury (TBI) Therapeutics market is characterized by distinct regional dominance and segment-specific growth drivers. North America, particularly the United States, is expected to maintain its leading position in terms of market share, driven by robust healthcare infrastructure, significant R&D investments, and a high prevalence of TBI cases. Key growth drivers in this region include strong government funding for neuroscience research and the presence of leading pharmaceutical and biotechnology companies actively engaged in TBI therapeutic development.
Application Dominance:
- Closed TBI: This segment is anticipated to dominate the market due to its higher incidence compared to other TBI types. Factors contributing to this dominance include the widespread occurrence of falls, traffic accidents, and assaults leading to closed head injuries.
- Diffuse TBI: As understanding of diffuse axonal injury (DAI) grows, so does the demand for targeted therapies. Advances in diagnostic imaging and a deeper comprehension of the widespread neural damage are propelling this segment.
- Local TBI: While less prevalent than diffuse injuries, specific localized TBI events still represent a significant market, particularly those requiring surgical intervention or targeted drug delivery to specific brain regions.
- Open TBI: Though less common, open TBI cases often present with severe injuries, necessitating advanced and often specialized therapeutic interventions, contributing to their market value.
- Primary TBI: This initial phase of injury is crucial for intervention, driving demand for acute care therapeutics aimed at preventing secondary injury cascades.
Type Dominance:
- Stem Cells: This segment holds immense promise and is projected to be a significant growth engine. The regenerative potential of stem cells for repairing damaged neural tissue and promoting neurogenesis is a key driver. Companies are investing heavily in clinical trials for various stem cell-based therapies.
- Erythropoietin: With its neuroprotective and anti-inflammatory properties, erythropoietin is gaining traction as a potential therapeutic agent for TBI, particularly in mitigating secondary injury cascades.
- Acetylcholinesterase Inhibitors: These are currently employed in managing cognitive and behavioral deficits post-TBI, contributing to the existing market share and expected to see continued use.
- Growth Hormone: While research is ongoing, growth hormone's potential role in promoting tissue repair and regeneration post-TBI positions it as an important future segment.
- Others: This broad category encompasses emerging drug candidates, gene therapies, and novel biomaterials that are under development and are expected to contribute to market diversification and growth.
The market's dominance in these segments is further supported by ongoing clinical trials and the development of innovative treatment protocols aimed at addressing the complex sequelae of TBI.
Traumatic Brain Injury Therapeutics Product Analysis
The Traumatic Brain Injury (TBI) Therapeutics market is witnessing a surge in product innovations focused on neuroprotection and regeneration. Advanced therapies, including stem cell-based treatments and novel drug candidates targeting neuroinflammation and excitotoxicity, are showcasing promising results in clinical trials. These products offer distinct competitive advantages by aiming to halt or reverse neuronal damage, a significant improvement over existing supportive care. Technological advancements in targeted drug delivery systems, such as nanoparticle-based formulations, are enhancing efficacy and minimizing off-target effects, making these treatments more viable for a wider range of TBI severity. The market fit for these innovations is strong, driven by the substantial unmet medical need for effective TBI treatments.
Key Drivers, Barriers & Challenges in Traumatic Brain Injury Therapeutics
Key Drivers: The Traumatic Brain Injury (TBI) Therapeutics market is propelled by several key factors. Significant advancements in neuroscience research, particularly in understanding the complex molecular and cellular mechanisms of TBI, are uncovering novel therapeutic targets. Increased global incidence of TBI, attributed to rising traffic accidents, falls, and sports-related injuries, creates a growing patient population. Growing government and private investments in R&D for neurological disorders, coupled with supportive regulatory pathways for promising therapies, further fuel market expansion. The development of advanced diagnostic tools for early and accurate TBI detection also contributes to the proactive initiation of therapeutic interventions.
Barriers & Challenges: Despite the promising outlook, the TBI therapeutics market faces considerable challenges. The complex pathophysiology of TBI, involving multiple injury cascades, makes developing single-target therapies difficult. High failure rates in clinical trials, particularly for drugs targeting acute TBI, represent a significant financial and temporal barrier. Stringent and lengthy regulatory approval processes add to the cost and time-to-market. Supply chain complexities for advanced biologics and cell therapies, alongside the high cost of novel treatments, can limit accessibility. Furthermore, the lack of standardized diagnostic criteria and outcome measures can complicate trial design and interpretation. Competitive pressures from established treatments and the ongoing need for extensive post-market surveillance also pose challenges.
Growth Drivers in the Traumatic Brain Injury Therapeutics Market
Growth in the Traumatic Brain Injury (TBI) Therapeutics market is significantly driven by escalating research into neuroprotection and regenerative medicine. Technological advancements enabling targeted drug delivery and the development of novel therapeutic modalities, such as stem cell therapies and gene therapies, are opening new avenues for treatment. The increasing global prevalence of TBI, arising from rising accident rates and an aging population susceptible to falls, is expanding the patient pool requiring effective interventions. Supportive government initiatives and increased funding for neurological research are accelerating the development pipeline. Furthermore, a growing emphasis on improving long-term patient outcomes and reducing the burden of disability post-TBI is driving demand for more effective therapeutic solutions.
Challenges Impacting Traumatic Brain Injury Therapeutics Growth
The Traumatic Brain Injury (TBI) Therapeutics market faces significant hurdles. The inherent complexity of TBI, involving intricate secondary injury cascades, makes developing effective interventions challenging, leading to a high attrition rate in clinical trials. Regulatory complexities, including stringent approval processes and the need for robust evidence of efficacy and safety, often prolong the time-to-market for new therapies. Supply chain issues, particularly for advanced biologics and cell-based treatments, can impact product availability and scalability. Furthermore, the high cost associated with research, development, and novel therapeutics can limit patient access and create significant financial barriers for healthcare systems and individuals, impacting overall market penetration. Competitive pressures from existing palliative care and the ongoing need for extensive long-term studies also add to the developmental challenges.
Key Players Shaping the Traumatic Brain Injury Therapeutics Market
- SFC Fluidics LLC
- Banayan Biomarkers Inc.
- BHR Pharma LLC
- Cerora Inc.
- ElMindA Ltd.
- Grace Laboratories LLC
- KeyNeurotek Pharmaceuticals AG
- Luoxis Diagnostics
- Neuro Assessment Systems
- Neurovive Pharmaceuticals AB
- Oxygen Biotherapeutics Inc.
- Phlogistix LLC
- Neurohealing Pharmaceuticals
- Neuren Pharmaceuticals Ltd.
- Remedy Pharmaceuticals Inc.
- Biodirection Inc.
- Brainscope Company Inc.
- TEVA Pharmaceutical Industries Ltd.
- Vasopharm
Significant Traumatic Brain Injury Therapeutics Industry Milestones
- 2019: Launch of advanced diagnostic platforms for early TBI detection, improving patient stratification.
- 2020: Breakthroughs in preclinical studies for novel neuroprotective agents targeting inflammatory pathways.
- 2021: Significant investment rounds for stem cell therapy companies focused on neurological repair.
- 2022: Initiation of Phase III clinical trials for promising erythropoietin-based TBI treatments.
- 2023: Regulatory approvals for new devices aimed at monitoring intracranial pressure in TBI patients.
- 2024: Emergence of strategic partnerships between pharmaceutical giants and biotech startups for TBI drug development.
- 2025 (Projected): Expected commencement of early-stage human trials for gene therapy approaches to TBI.
- 2026 (Projected): Anticipated regulatory review of first-in-class regenerative therapies for severe TBI.
- 2027 (Projected): Expansion of clinical trials for combination therapies to address the multifaceted nature of TBI.
- 2028 (Projected): Introduction of new biomarkers for predicting TBI recovery trajectories.
- 2029 (Projected): Growing adoption of AI-driven platforms for TBI diagnosis and personalized treatment planning.
- 2030 (Projected): Advancements in delivery systems for enhanced blood-brain barrier penetration of therapeutics.
- 2031 (Projected): Increased focus on rehabilitative therapeutics that complement pharmacological interventions.
- 2032 (Projected): Development of standardized protocols for long-term TBI management and follow-up.
- 2033 (Projected): Significant market penetration of regenerative and neuroprotective therapies for TBI.
Future Outlook for Traumatic Brain Injury Therapeutics Market
The future outlook for the Traumatic Brain Injury (TBI) Therapeutics market is highly optimistic, driven by a confluence of scientific innovation and increasing healthcare expenditure. Strategic opportunities lie in the development of personalized medicine approaches, leveraging advanced diagnostics and biomarkers to tailor treatments to individual patient needs. The continued exploration of regenerative medicine, particularly stem cell and gene therapies, holds immense potential to revolutionize TBI recovery. Furthermore, advancements in drug delivery systems will enhance the efficacy and safety of therapeutic interventions. The growing global awareness and incidence of TBI will sustain demand, while ongoing research into neuroprotection and neuroinflammation will unlock new therapeutic targets, paving the way for more effective and comprehensive treatment options that aim for significant functional restoration and improved quality of life for patients.
Traumatic Brain Injury Therapeutics Segmentation
-
1. Application
- 1.1. Local TBI
- 1.2. Open TBI
- 1.3. Closed TBI
- 1.4. Diffuse TBI
- 1.5. Primary TBI
- 1.6. Others
-
2. Type
- 2.1. Acetylcholinesterase Inhibitors
- 2.2. Erythropoietin
- 2.3. Growth Hormone
- 2.4. Stem Cells
- 2.5. Others
Traumatic Brain Injury Therapeutics Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific

Traumatic Brain Injury Therapeutics Regional Market Share

Geographic Coverage of Traumatic Brain Injury Therapeutics
Traumatic Brain Injury Therapeutics REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 3.5% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Local TBI
- 5.1.2. Open TBI
- 5.1.3. Closed TBI
- 5.1.4. Diffuse TBI
- 5.1.5. Primary TBI
- 5.1.6. Others
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. Acetylcholinesterase Inhibitors
- 5.2.2. Erythropoietin
- 5.2.3. Growth Hormone
- 5.2.4. Stem Cells
- 5.2.5. Others
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Local TBI
- 6.1.2. Open TBI
- 6.1.3. Closed TBI
- 6.1.4. Diffuse TBI
- 6.1.5. Primary TBI
- 6.1.6. Others
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. Acetylcholinesterase Inhibitors
- 6.2.2. Erythropoietin
- 6.2.3. Growth Hormone
- 6.2.4. Stem Cells
- 6.2.5. Others
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Local TBI
- 7.1.2. Open TBI
- 7.1.3. Closed TBI
- 7.1.4. Diffuse TBI
- 7.1.5. Primary TBI
- 7.1.6. Others
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. Acetylcholinesterase Inhibitors
- 7.2.2. Erythropoietin
- 7.2.3. Growth Hormone
- 7.2.4. Stem Cells
- 7.2.5. Others
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Local TBI
- 8.1.2. Open TBI
- 8.1.3. Closed TBI
- 8.1.4. Diffuse TBI
- 8.1.5. Primary TBI
- 8.1.6. Others
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. Acetylcholinesterase Inhibitors
- 8.2.2. Erythropoietin
- 8.2.3. Growth Hormone
- 8.2.4. Stem Cells
- 8.2.5. Others
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Local TBI
- 9.1.2. Open TBI
- 9.1.3. Closed TBI
- 9.1.4. Diffuse TBI
- 9.1.5. Primary TBI
- 9.1.6. Others
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. Acetylcholinesterase Inhibitors
- 9.2.2. Erythropoietin
- 9.2.3. Growth Hormone
- 9.2.4. Stem Cells
- 9.2.5. Others
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Traumatic Brain Injury Therapeutics Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Local TBI
- 10.1.2. Open TBI
- 10.1.3. Closed TBI
- 10.1.4. Diffuse TBI
- 10.1.5. Primary TBI
- 10.1.6. Others
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. Acetylcholinesterase Inhibitors
- 10.2.2. Erythropoietin
- 10.2.3. Growth Hormone
- 10.2.4. Stem Cells
- 10.2.5. Others
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 SFC Fluidics LLC
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Banayan Biomarkers Inc.
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 BHR Pharma LLC
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Cerora Inc.
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 ElMindA Ltd.
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Grace Laboratories LLC
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 KeyNeurotek Pharmaceuticals AG
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Luoxis Diagnostics
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Neuro Assessment Systems
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Neurovive Pharmaceuticals AB
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Oxygen Biotherapeutics Inc.
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Phlogistix LLC
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Neurohealing Pharmaceuticals
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Neuren Pharmaceuticals Ltd.
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Remedy Pharmaceuticals Inc.
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.16 Biodirection Inc.
- 11.2.16.1. Overview
- 11.2.16.2. Products
- 11.2.16.3. SWOT Analysis
- 11.2.16.4. Recent Developments
- 11.2.16.5. Financials (Based on Availability)
- 11.2.17 Brainscope Company Inc.
- 11.2.17.1. Overview
- 11.2.17.2. Products
- 11.2.17.3. SWOT Analysis
- 11.2.17.4. Recent Developments
- 11.2.17.5. Financials (Based on Availability)
- 11.2.18 TEVA Pharmaceutical Industries Ltd.
- 11.2.18.1. Overview
- 11.2.18.2. Products
- 11.2.18.3. SWOT Analysis
- 11.2.18.4. Recent Developments
- 11.2.18.5. Financials (Based on Availability)
- 11.2.19 Vasopharm
- 11.2.19.1. Overview
- 11.2.19.2. Products
- 11.2.19.3. SWOT Analysis
- 11.2.19.4. Recent Developments
- 11.2.19.5. Financials (Based on Availability)
- 11.2.1 SFC Fluidics LLC
List of Figures
- Figure 1: Global Traumatic Brain Injury Therapeutics Revenue Breakdown (million, %) by Region 2025 & 2033
- Figure 2: North America Traumatic Brain Injury Therapeutics Revenue (million), by Application 2025 & 2033
- Figure 3: North America Traumatic Brain Injury Therapeutics Revenue Share (%), by Application 2025 & 2033
- Figure 4: North America Traumatic Brain Injury Therapeutics Revenue (million), by Type 2025 & 2033
- Figure 5: North America Traumatic Brain Injury Therapeutics Revenue Share (%), by Type 2025 & 2033
- Figure 6: North America Traumatic Brain Injury Therapeutics Revenue (million), by Country 2025 & 2033
- Figure 7: North America Traumatic Brain Injury Therapeutics Revenue Share (%), by Country 2025 & 2033
- Figure 8: South America Traumatic Brain Injury Therapeutics Revenue (million), by Application 2025 & 2033
- Figure 9: South America Traumatic Brain Injury Therapeutics Revenue Share (%), by Application 2025 & 2033
- Figure 10: South America Traumatic Brain Injury Therapeutics Revenue (million), by Type 2025 & 2033
- Figure 11: South America Traumatic Brain Injury Therapeutics Revenue Share (%), by Type 2025 & 2033
- Figure 12: South America Traumatic Brain Injury Therapeutics Revenue (million), by Country 2025 & 2033
- Figure 13: South America Traumatic Brain Injury Therapeutics Revenue Share (%), by Country 2025 & 2033
- Figure 14: Europe Traumatic Brain Injury Therapeutics Revenue (million), by Application 2025 & 2033
- Figure 15: Europe Traumatic Brain Injury Therapeutics Revenue Share (%), by Application 2025 & 2033
- Figure 16: Europe Traumatic Brain Injury Therapeutics Revenue (million), by Type 2025 & 2033
- Figure 17: Europe Traumatic Brain Injury Therapeutics Revenue Share (%), by Type 2025 & 2033
- Figure 18: Europe Traumatic Brain Injury Therapeutics Revenue (million), by Country 2025 & 2033
- Figure 19: Europe Traumatic Brain Injury Therapeutics Revenue Share (%), by Country 2025 & 2033
- Figure 20: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue (million), by Application 2025 & 2033
- Figure 21: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue Share (%), by Application 2025 & 2033
- Figure 22: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue (million), by Type 2025 & 2033
- Figure 23: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue Share (%), by Type 2025 & 2033
- Figure 24: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue (million), by Country 2025 & 2033
- Figure 25: Middle East & Africa Traumatic Brain Injury Therapeutics Revenue Share (%), by Country 2025 & 2033
- Figure 26: Asia Pacific Traumatic Brain Injury Therapeutics Revenue (million), by Application 2025 & 2033
- Figure 27: Asia Pacific Traumatic Brain Injury Therapeutics Revenue Share (%), by Application 2025 & 2033
- Figure 28: Asia Pacific Traumatic Brain Injury Therapeutics Revenue (million), by Type 2025 & 2033
- Figure 29: Asia Pacific Traumatic Brain Injury Therapeutics Revenue Share (%), by Type 2025 & 2033
- Figure 30: Asia Pacific Traumatic Brain Injury Therapeutics Revenue (million), by Country 2025 & 2033
- Figure 31: Asia Pacific Traumatic Brain Injury Therapeutics Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Region 2020 & 2033
- Table 2: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 3: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 4: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Region 2020 & 2033
- Table 5: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 6: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 7: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Country 2020 & 2033
- Table 8: United States Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 9: Canada Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 10: Mexico Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 11: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 12: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 13: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Country 2020 & 2033
- Table 14: Brazil Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 15: Argentina Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 16: Rest of South America Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 17: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 18: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 19: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Country 2020 & 2033
- Table 20: United Kingdom Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 21: Germany Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 22: France Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 23: Italy Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 24: Spain Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 25: Russia Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 26: Benelux Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 27: Nordics Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 28: Rest of Europe Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 29: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 30: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 31: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Country 2020 & 2033
- Table 32: Turkey Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 33: Israel Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 34: GCC Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 35: North Africa Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 36: South Africa Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 37: Rest of Middle East & Africa Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 38: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Application 2020 & 2033
- Table 39: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Type 2020 & 2033
- Table 40: Global Traumatic Brain Injury Therapeutics Revenue million Forecast, by Country 2020 & 2033
- Table 41: China Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 42: India Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 43: Japan Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 44: South Korea Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 45: ASEAN Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 46: Oceania Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
- Table 47: Rest of Asia Pacific Traumatic Brain Injury Therapeutics Revenue (million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Traumatic Brain Injury Therapeutics?
The projected CAGR is approximately 3.5%.
2. Which companies are prominent players in the Traumatic Brain Injury Therapeutics?
Key companies in the market include SFC Fluidics LLC, Banayan Biomarkers Inc., BHR Pharma LLC, Cerora Inc., ElMindA Ltd., Grace Laboratories LLC, KeyNeurotek Pharmaceuticals AG, Luoxis Diagnostics, Neuro Assessment Systems, Neurovive Pharmaceuticals AB, Oxygen Biotherapeutics Inc., Phlogistix LLC, Neurohealing Pharmaceuticals, Neuren Pharmaceuticals Ltd., Remedy Pharmaceuticals Inc., Biodirection Inc., Brainscope Company Inc., TEVA Pharmaceutical Industries Ltd., Vasopharm.
3. What are the main segments of the Traumatic Brain Injury Therapeutics?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 1261 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3950.00, USD 5925.00, and USD 7900.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Traumatic Brain Injury Therapeutics," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Traumatic Brain Injury Therapeutics report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Traumatic Brain Injury Therapeutics?
To stay informed about further developments, trends, and reports in the Traumatic Brain Injury Therapeutics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
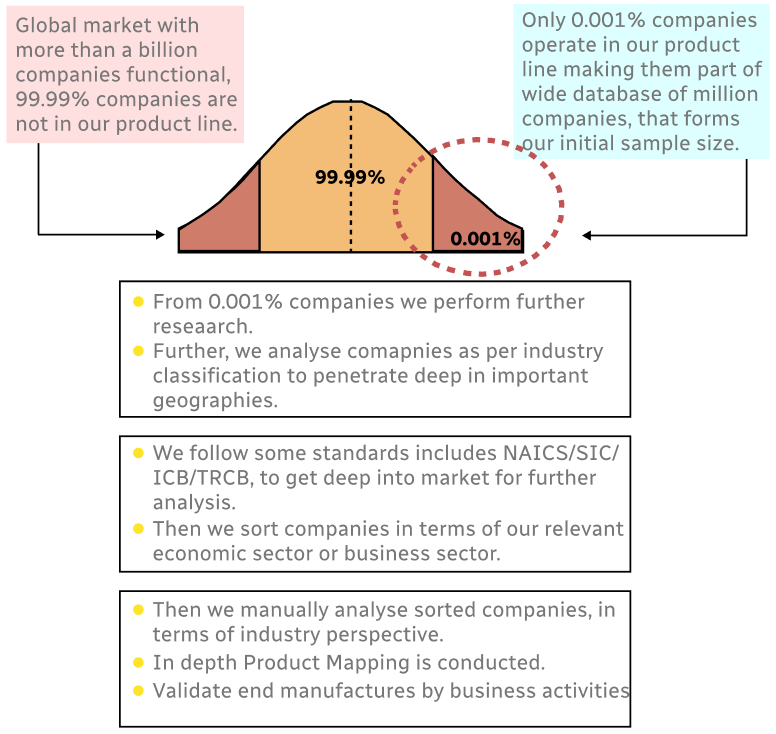
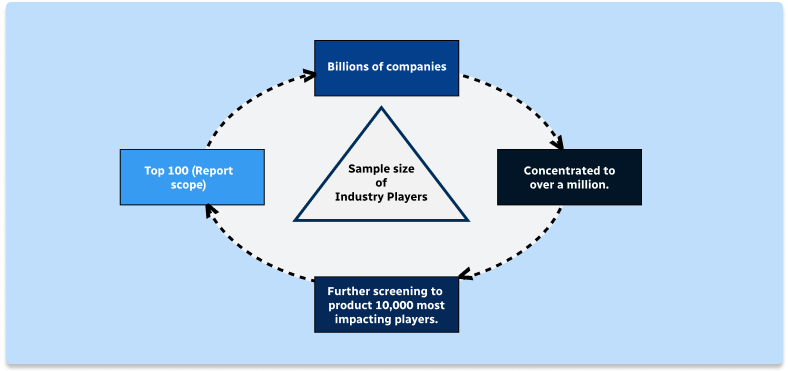
Step 1 - Identification of Relevant Samples Size from Population Database
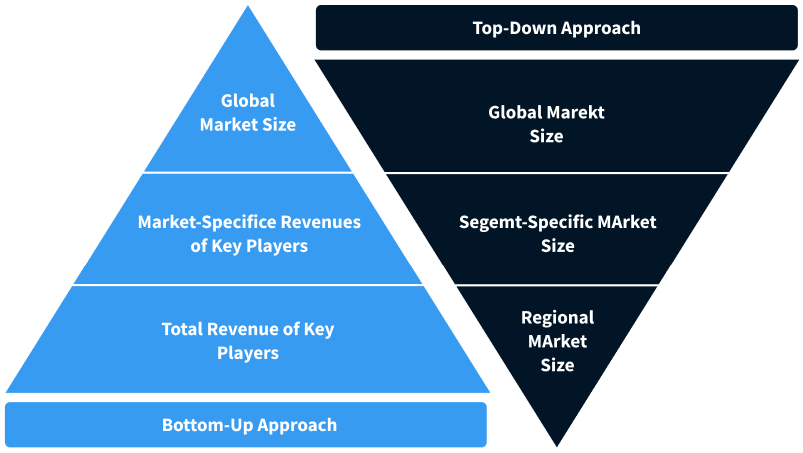
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


