Key Insights
The global Biopharmaceutical Third Party Logistics (3PL) market is experiencing robust growth, projected to reach a substantial market size of approximately $120 billion by 2025, with a compelling Compound Annual Growth Rate (CAGR) of around 10.5% anticipated through 2033. This expansion is fundamentally driven by the increasing complexity of biopharmaceutical supply chains, stringent regulatory requirements for temperature-controlled and secure transportation of sensitive biologics, and the growing global demand for advanced therapies. Key industry players are investing heavily in specialized infrastructure, advanced technologies like IoT for real-time tracking and monitoring, and innovative cold chain solutions to meet these evolving needs. The market is segmenting into Transportation, Warehousing, and Value-added Services, with each playing a critical role in ensuring product integrity and timely delivery from manufacturers to distributors and ultimately to healthcare providers and patients.
The market's positive trajectory is further fueled by strategic partnerships and collaborations between biopharmaceutical companies and specialized 3PL providers, allowing manufacturers to focus on core R&D and production while outsourcing logistics complexities. Trends such as the rise of personalized medicine, the increasing prevalence of biologics and vaccines requiring specialized handling, and the expansion of biopharmaceutical manufacturing into emerging markets are creating significant opportunities. However, challenges such as high operational costs associated with maintaining cold chains, the need for skilled labor in specialized logistics, and the ever-present threat of supply chain disruptions due to geopolitical events or natural disasters, necessitate continuous innovation and resilience from 3PL providers. Key regions like Asia Pacific and Europe are expected to witness particularly strong growth due to expanding biopharmaceutical production hubs and increasing healthcare expenditure.
This in-depth report provides a dynamic, SEO-optimized analysis of the Biopharmaceutical Third Party Logistics (3PL) market, essential for stakeholders seeking to navigate this complex and rapidly evolving sector. Leveraging high-volume keywords such as "biopharmaceutical logistics," "cold chain logistics," "pharmaceutical supply chain," "biologics transport," and "specialty pharma logistics," this report aims to enhance search rankings and engage industry audiences. The study meticulously covers the period from 2019 to 2033, with a base year of 2025, providing robust insights into market structure, competitive landscape, emerging trends, dominant segments, key players, and future outlook.
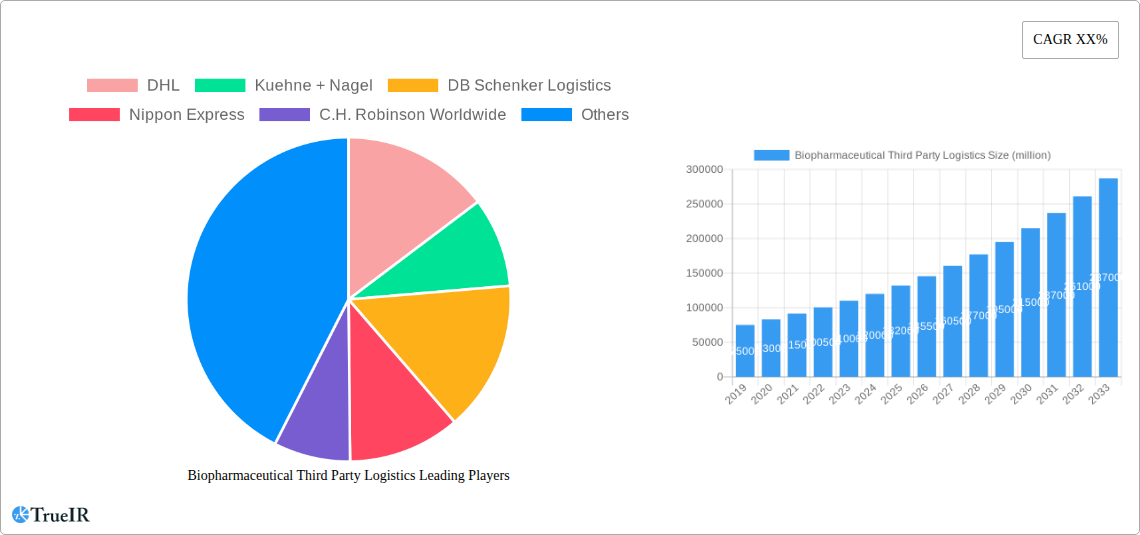
Biopharmaceutical Third Party Logistics Market Structure & Competitive Landscape
The biopharmaceutical third party logistics market is characterized by a moderate to high level of concentration, driven by the significant capital investment required for specialized infrastructure, advanced technology, and stringent regulatory compliance. Key innovation drivers include the demand for temperature-controlled transportation (cold chain), serialization and track-and-trace capabilities, and the increasing complexity of biologic drug distribution. Regulatory impacts, such as Good Distribution Practices (GDP) and evolving pharmaceutical import/export laws, significantly shape market entry and operational strategies. Product substitutes, while limited for highly specialized biopharmaceutical products, can include in-house logistics capabilities for larger, established companies. End-user segmentation reveals a dominant share held by Biopharmaceutical Manufacturers, followed by Biopharmaceutical Distributors. Mergers and Acquisitions (M&A) trends are notable, with approximately 50 significant M&A deals observed between 2019 and 2024, signaling a consolidation phase aimed at expanding service portfolios and geographic reach. Key M&A drivers include the acquisition of specialized cold chain capabilities and technology-driven solutions.
- Market Concentration: Moderate to High
- Innovation Drivers: Cold Chain Technology, Serialization, Biologics Handling, Real-time Visibility
- Regulatory Impacts: GDP Compliance, FDA Regulations, EMA Guidelines, Import/Export Controls
- Product Substitutes: In-house Logistics, Integrated Supply Chain Solutions
- End-User Segmentation: Biopharmaceutical Manufacturers (xx%), Biopharmaceutical Distributors (xx%), Others (xx%)
- M&A Trends: Increasing consolidation, focus on specialized services and technology acquisition.
Biopharmaceutical Third Party Logistics Market Trends & Opportunities
The Biopharmaceutical Third Party Logistics market is projected to witness substantial growth, driven by the escalating global demand for biopharmaceuticals, including biologics, vaccines, and cell and gene therapies. The market size is anticipated to expand from an estimated $XX billion in 2025 to over $XX billion by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of approximately XX% during the forecast period (2025-2033). Technological shifts are at the forefront of this evolution, with the widespread adoption of advanced cold chain solutions, including active temperature-controlled containers and real-time monitoring systems, becoming a critical differentiator. The integration of Artificial Intelligence (AI) and Machine Learning (ML) for optimizing route planning, inventory management, and demand forecasting is also gaining traction. Consumer preferences, primarily dictated by biopharmaceutical manufacturers and distributors, are increasingly leaning towards logistics providers that offer end-to-end supply chain visibility, robust risk management, and demonstrable compliance with stringent regulatory standards. Competitive dynamics are intensifying, with a clear trend towards specialization in handling temperature-sensitive and high-value products. Opportunities abound in emerging markets with growing biopharmaceutical production, as well as in the specialized logistics requirements for novel therapies like personalized medicine and gene therapies. The increasing outsourcing of logistics by biopharmaceutical companies, driven by the need to focus on core competencies and manage complex supply chains, further fuels market expansion. Furthermore, the growing emphasis on sustainable logistics practices, including reducing carbon footprints in transportation and warehousing, presents a significant emerging trend and opportunity for forward-thinking 3PL providers. The development of advanced packaging solutions that maintain product integrity under extreme temperature variations is also a key trend. The rise of biosimilars and the expanding global pharmaceutical market contribute to sustained demand for efficient and reliable logistics services. The market penetration rate for specialized biopharmaceutical 3PL services is expected to increase as more companies recognize the value of expert handling and compliance.
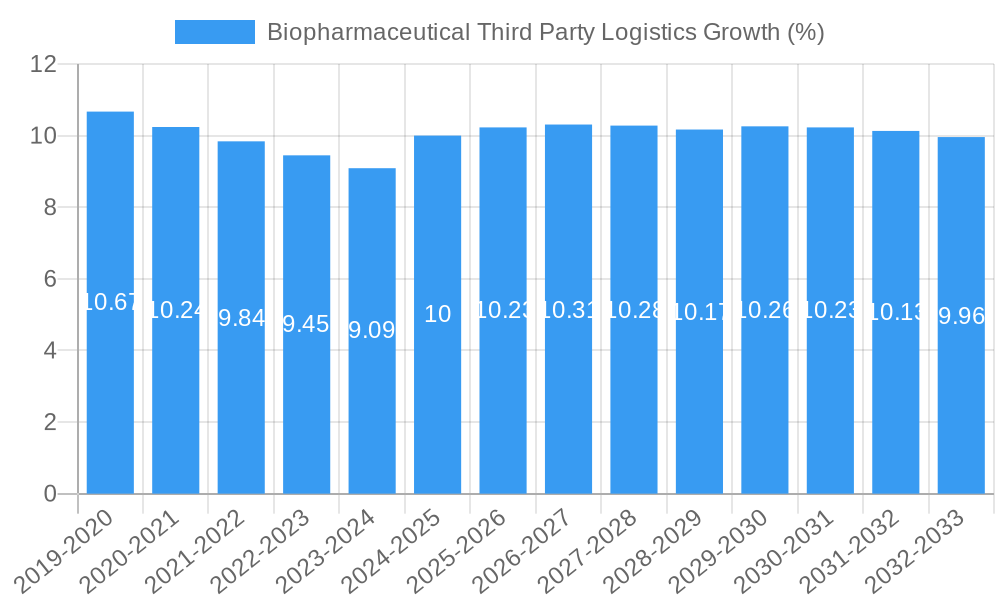
Dominant Markets & Segments in Biopharmaceutical Third Party Logistics
The Transportation segment is expected to remain the largest and most dominant within the Biopharmaceutical Third Party Logistics market, driven by the critical need for secure and temperature-controlled movement of highly sensitive biopharmaceutical products across global supply chains. This dominance is fueled by the increasing complexity of global distribution networks and the rising volume of temperature-sensitive biologics and vaccines. Within the Application segment, Biopharmaceutical Manufacturers represent the leading end-user, accounting for the largest share of demand for 3PL services as they increasingly outsource their logistics operations to focus on research, development, and production. Regional dominance is projected for North America and Europe, owing to the mature biopharmaceutical industries, presence of major research institutions and manufacturing hubs, and stringent regulatory frameworks that necessitate specialized logistics expertise. Key growth drivers in these regions include robust government support for the pharmaceutical sector, advanced healthcare infrastructure, and high adoption rates of innovative logistics technologies. Asia-Pacific is emerging as a significant growth region, driven by expanding biopharmaceutical manufacturing capabilities and increasing healthcare expenditure.
- Dominant Segment (Type): Transportation (XX% market share expected by 2025)
- Key Growth Drivers: Global distribution networks, cold chain requirements for biologics and vaccines, specialized transport solutions.
- Dominant Segment (Application): Biopharmaceutical Manufacturers (XX% market share expected by 2025)
- Key Growth Drivers: Outsourcing of logistics for core competency focus, increasing complexity of product portfolios, need for specialized handling.
- Dominant Regions:
- North America: Established biopharmaceutical market, stringent regulatory environment, high demand for specialized logistics.
- Europe: Strong research and manufacturing base, robust regulatory framework (GDP), significant demand for cold chain logistics.
- Asia-Pacific: Rapidly growing biopharmaceutical sector, increasing healthcare investment, expanding manufacturing capabilities.
Biopharmaceutical Third Party Logistics Product Analysis
The Biopharmaceutical Third Party Logistics market's product offering is characterized by highly specialized solutions focused on maintaining product integrity and regulatory compliance throughout the supply chain. Key product innovations include advanced temperature-controlled packaging, real-time GPS tracking and temperature monitoring systems, and serialization solutions for enhanced traceability. These offerings are crucial for the safe and efficient transport of biologics, vaccines, and other temperature-sensitive pharmaceuticals. Competitive advantages are derived from providers' ability to offer end-to-end supply chain management, integrated IT solutions for visibility, and expertise in navigating complex international regulations. The market fit is dictated by the unique demands of biopharmaceutical products, where temperature excursions or breaches in security can lead to significant product loss and patient harm.
Key Drivers, Barriers & Challenges in Biopharmaceutical Third Party Logistics
Key Drivers: The biopharmaceutical third party logistics market is propelled by several critical factors. Technological advancements in cold chain management and real-time monitoring are paramount, enabling the safe transport of sensitive biologics. The increasing outsourcing trend by biopharmaceutical companies, driven by a desire to focus on core competencies and manage escalating supply chain complexity, is a significant economic driver. Furthermore, evolving global regulations, such as Good Distribution Practices (GDP), mandate specialized logistics capabilities, creating demand for expert 3PL providers. The expanding global market for biologics and novel therapies also fuels growth.
Barriers & Challenges: Significant barriers include the high capital investment required for specialized infrastructure (e.g., temperature-controlled warehouses and fleets) and technology. Stringent and evolving regulatory hurdles across different regions pose a constant challenge, requiring continuous adaptation and compliance. Supply chain disruptions, exacerbated by geopolitical events and pandemics, present considerable risks. Intense competitive pressures from both established logistics giants and niche specialized providers can impact pricing and market share. The complexity of managing global supply chains for high-value, temperature-sensitive products, including the risk of temperature excursions, remains a persistent challenge.
Growth Drivers in the Biopharmaceutical Third Party Logistics Market
Key growth drivers for the biopharmaceutical third party logistics market are deeply rooted in technological innovation, economic expansion, and evolving regulatory landscapes. The relentless advancement in cold chain technologies, from passive insulation to active temperature-controlled systems and real-time monitoring, is crucial for ensuring the integrity of temperature-sensitive biopharmaceuticals. Economically, the burgeoning global market for biologics, vaccines, and advanced therapies, coupled with the strategic decision of many biopharmaceutical manufacturers to outsource their logistics operations, creates substantial demand. Regulatory tailwinds, such as the increasing emphasis on Good Distribution Practices (GDP) and serialization requirements, necessitate specialized logistics expertise, thereby driving the adoption of third-party solutions. The growing demand for personalized medicine and the expansion of clinical trials globally also contribute significantly to the need for flexible and reliable logistics networks.
Challenges Impacting Biopharmaceutical Third Party Logistics Growth
The growth of the biopharmaceutical third party logistics market is not without its obstacles. Regulatory complexities and the continuous evolution of compliance requirements across diverse international markets pose a significant challenge, demanding constant vigilance and adaptation from 3PL providers. Supply chain issues, including the inherent risks associated with transporting high-value, temperature-sensitive products, can lead to costly product loss and reputational damage. Competitive pressures are intensifying, with both established players and emerging niche providers vying for market share, often leading to price sensitivity. The substantial capital investment required for specialized infrastructure, such as temperature-controlled warehousing and advanced transportation fleets, can also act as a barrier to entry and expansion. Furthermore, the ongoing need for skilled labor with expertise in handling biopharmaceuticals adds another layer of operational complexity.
Key Players Shaping the Biopharmaceutical Third Party Logistics Market
- DHL
- Kuehne + Nagel
- DB Schenker Logistics
- Nippon Express
- C.H. Robinson Worldwide
- UPS Supply Chain Solutions
- DSV
- Sinotrans
- CEVA Logistics
- Expeditors International of Washington
- Dachser
- Panalpina
- GEODIS
- Toll Holdings
- J.B. Hunt (JBI, DCS & ICS)
- Hitachi Transport System
- XPO Logistics
- GEFCO
- Yusen Logistics
- Agility
Significant Biopharmaceutical Third Party Logistics Industry Milestones
- 2019: Increased investment in AI-driven route optimization for cold chain logistics by major players.
- 2020: Surge in demand for temperature-controlled transport and warehousing due to global vaccine distribution efforts.
- 2021: Expansion of serialization and track-and-trace capabilities across the supply chain to combat counterfeit drugs.
- 2022: Growing focus on sustainable logistics practices, including reduced carbon footprints in biopharmaceutical transportation.
- 2023: Acquisitions of specialized cold chain providers by larger logistics companies to enhance service offerings.
- 2024: Increased adoption of blockchain technology for enhanced supply chain transparency and security in biopharmaceutical logistics.
Future Outlook for Biopharmaceutical Third Party Logistics Market
- 2019: Increased investment in AI-driven route optimization for cold chain logistics by major players.
- 2020: Surge in demand for temperature-controlled transport and warehousing due to global vaccine distribution efforts.
- 2021: Expansion of serialization and track-and-trace capabilities across the supply chain to combat counterfeit drugs.
- 2022: Growing focus on sustainable logistics practices, including reduced carbon footprints in biopharmaceutical transportation.
- 2023: Acquisitions of specialized cold chain providers by larger logistics companies to enhance service offerings.
- 2024: Increased adoption of blockchain technology for enhanced supply chain transparency and security in biopharmaceutical logistics.
Future Outlook for Biopharmaceutical Third Party Logistics Market
The future outlook for the Biopharmaceutical Third Party Logistics market is exceptionally promising, driven by a confluence of factors including the projected growth in biologics, vaccines, and advanced therapies. Strategic opportunities lie in the continued investment in cutting-edge cold chain technologies, robust IT infrastructure for end-to-end visibility, and the expansion into emerging markets with burgeoning biopharmaceutical sectors. 3PL providers that can demonstrate unparalleled expertise in regulatory compliance, risk mitigation, and sustainable logistics practices will be best positioned to capitalize on the increasing demand for specialized and reliable supply chain solutions. The market potential is further amplified by the ongoing trend of outsourcing, allowing biopharmaceutical companies to focus on innovation while entrusting their complex logistics needs to expert partners.
Biopharmaceutical Third Party Logistics Segmentation
-
1. Application
- 1.1. Biopharmaceutical Manufacturers
- 1.2. Biopharmaceutical Distributors
- 1.3. Others
-
2. Types
- 2.1. Transportation
- 2.2. Warehousing
- 2.3. Value-added Services
- 2.4. Other
Biopharmaceutical Third Party Logistics Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
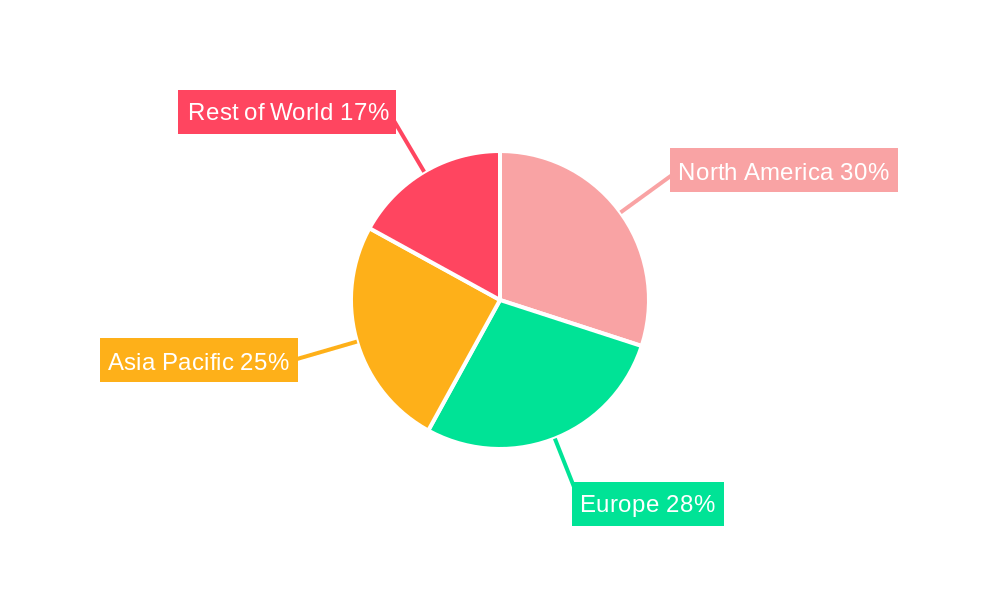
Biopharmaceutical Third Party Logistics REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Biopharmaceutical Manufacturers
- 5.1.2. Biopharmaceutical Distributors
- 5.1.3. Others
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Transportation
- 5.2.2. Warehousing
- 5.2.3. Value-added Services
- 5.2.4. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Biopharmaceutical Manufacturers
- 6.1.2. Biopharmaceutical Distributors
- 6.1.3. Others
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Transportation
- 6.2.2. Warehousing
- 6.2.3. Value-added Services
- 6.2.4. Other
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Biopharmaceutical Manufacturers
- 7.1.2. Biopharmaceutical Distributors
- 7.1.3. Others
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Transportation
- 7.2.2. Warehousing
- 7.2.3. Value-added Services
- 7.2.4. Other
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Biopharmaceutical Manufacturers
- 8.1.2. Biopharmaceutical Distributors
- 8.1.3. Others
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Transportation
- 8.2.2. Warehousing
- 8.2.3. Value-added Services
- 8.2.4. Other
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Biopharmaceutical Manufacturers
- 9.1.2. Biopharmaceutical Distributors
- 9.1.3. Others
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Transportation
- 9.2.2. Warehousing
- 9.2.3. Value-added Services
- 9.2.4. Other
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Biopharmaceutical Third Party Logistics Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Biopharmaceutical Manufacturers
- 10.1.2. Biopharmaceutical Distributors
- 10.1.3. Others
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Transportation
- 10.2.2. Warehousing
- 10.2.3. Value-added Services
- 10.2.4. Other
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 DHL
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Kuehne + Nagel
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 DB Schenker Logistics
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Nippon Express
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 C.H. Robinson Worldwide
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 UPS Supply Chain Solutions
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 DSV
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Sinotrans
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 CEVA Logistics
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Expeditors International of Washington
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Dachser
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Panalpina
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 GEODIS
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Toll Holdings
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 J.B. Hunt (JBI
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.16 DCS & ICS)
- 11.2.16.1. Overview
- 11.2.16.2. Products
- 11.2.16.3. SWOT Analysis
- 11.2.16.4. Recent Developments
- 11.2.16.5. Financials (Based on Availability)
- 11.2.17 Hitachi Transport System
- 11.2.17.1. Overview
- 11.2.17.2. Products
- 11.2.17.3. SWOT Analysis
- 11.2.17.4. Recent Developments
- 11.2.17.5. Financials (Based on Availability)
- 11.2.18 XPO Logistics
- 11.2.18.1. Overview
- 11.2.18.2. Products
- 11.2.18.3. SWOT Analysis
- 11.2.18.4. Recent Developments
- 11.2.18.5. Financials (Based on Availability)
- 11.2.19 GEFCO
- 11.2.19.1. Overview
- 11.2.19.2. Products
- 11.2.19.3. SWOT Analysis
- 11.2.19.4. Recent Developments
- 11.2.19.5. Financials (Based on Availability)
- 11.2.20 Yusen Logistics
- 11.2.20.1. Overview
- 11.2.20.2. Products
- 11.2.20.3. SWOT Analysis
- 11.2.20.4. Recent Developments
- 11.2.20.5. Financials (Based on Availability)
- 11.2.21 Agility
- 11.2.21.1. Overview
- 11.2.21.2. Products
- 11.2.21.3. SWOT Analysis
- 11.2.21.4. Recent Developments
- 11.2.21.5. Financials (Based on Availability)
- 11.2.1 DHL
List of Figures
- Figure 1: Global Biopharmaceutical Third Party Logistics Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Biopharmaceutical Third Party Logistics Revenue (million), by Application 2024 & 2032
- Figure 3: North America Biopharmaceutical Third Party Logistics Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Biopharmaceutical Third Party Logistics Revenue (million), by Types 2024 & 2032
- Figure 5: North America Biopharmaceutical Third Party Logistics Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Biopharmaceutical Third Party Logistics Revenue (million), by Country 2024 & 2032
- Figure 7: North America Biopharmaceutical Third Party Logistics Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Biopharmaceutical Third Party Logistics Revenue (million), by Application 2024 & 2032
- Figure 9: South America Biopharmaceutical Third Party Logistics Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Biopharmaceutical Third Party Logistics Revenue (million), by Types 2024 & 2032
- Figure 11: South America Biopharmaceutical Third Party Logistics Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Biopharmaceutical Third Party Logistics Revenue (million), by Country 2024 & 2032
- Figure 13: South America Biopharmaceutical Third Party Logistics Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Biopharmaceutical Third Party Logistics Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Biopharmaceutical Third Party Logistics Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Biopharmaceutical Third Party Logistics Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Biopharmaceutical Third Party Logistics Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Biopharmaceutical Third Party Logistics Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Biopharmaceutical Third Party Logistics Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Biopharmaceutical Third Party Logistics Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Biopharmaceutical Third Party Logistics Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Biopharmaceutical Third Party Logistics Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Biopharmaceutical Third Party Logistics Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Biopharmaceutical Third Party Logistics Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Biopharmaceutical Third Party Logistics Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Biopharmaceutical Third Party Logistics Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Biopharmaceutical Third Party Logistics Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Biopharmaceutical Third Party Logistics Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Biopharmaceutical Third Party Logistics?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Biopharmaceutical Third Party Logistics?
Key companies in the market include DHL, Kuehne + Nagel, DB Schenker Logistics, Nippon Express, C.H. Robinson Worldwide, UPS Supply Chain Solutions, DSV, Sinotrans, CEVA Logistics, Expeditors International of Washington, Dachser, Panalpina, GEODIS, Toll Holdings, J.B. Hunt (JBI, DCS & ICS), Hitachi Transport System, XPO Logistics, GEFCO, Yusen Logistics, Agility.
3. What are the main segments of the Biopharmaceutical Third Party Logistics?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3350.00, USD 5025.00, and USD 6700.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Biopharmaceutical Third Party Logistics," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Biopharmaceutical Third Party Logistics report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Biopharmaceutical Third Party Logistics?
To stay informed about further developments, trends, and reports in the Biopharmaceutical Third Party Logistics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


