Key Insights
The Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market is experiencing robust growth, projected to reach \$243.29 million in 2025 and maintain a Compound Annual Growth Rate (CAGR) of 6.41% from 2025 to 2033. This expansion is driven by several key factors. The increasing complexity of drug development, coupled with rising R&D costs, incentivizes pharmaceutical companies to outsource aspects of the process to specialized CDMOs. This allows them to focus on core competencies, accelerate time-to-market, and manage risk more effectively. Furthermore, the growing demand for biologics and advanced therapies, including cell and gene therapies, fuels the need for CDMOs with specialized expertise and infrastructure. The market is segmented by research phase (pre-clinical to Phase IV) and service type (API manufacturing, HPAPI, various dosage formulations, and secondary packaging), reflecting the diverse range of services offered by CDMOs. Geographic growth is expected across regions, with North America and Europe likely to maintain significant market share, while Asia-Pacific is poised for substantial growth due to increasing domestic pharmaceutical production and a growing middle class. The competitive landscape is highly fragmented, with numerous established players and emerging companies vying for market share. However, consolidation through mergers and acquisitions is anticipated as larger CDMOs seek to expand their service offerings and geographic reach.
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market Size (In Million)

The market's growth trajectory will likely be influenced by several factors in the coming years. Technological advancements, such as automation and AI-driven process optimization, will enhance efficiency and reduce costs within the CDMO sector. Regulatory changes and evolving industry standards will also play a significant role, potentially shaping the landscape through increased compliance requirements. Furthermore, strategic partnerships and collaborations between CDMOs and pharmaceutical companies are expected to become more prevalent, fostering innovation and accelerating the development of novel therapeutics. Despite potential challenges, the long-term outlook for the CDMO market remains positive, driven by sustained demand for outsourcing services and the continuous evolution of the pharmaceutical industry.
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Company Market Share

Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market: A Comprehensive Report (2019-2033)
This dynamic report provides a comprehensive analysis of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market, offering invaluable insights for stakeholders across the pharmaceutical and biotechnology industries. Leveraging extensive data from 2019-2024 (historical period), with a base year of 2025 and forecast extending to 2033, this report unveils key trends, growth drivers, and challenges shaping this rapidly evolving market. The study period covers a crucial period of market evolution, capturing significant shifts in technology, regulatory landscapes, and competitive dynamics. With a detailed examination of key segments and leading players, including LSK Global Pharma Service Co Ltd, Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc), and many more, this report is an indispensable resource for strategic decision-making. The report's market size is predicted at xx Million in 2025 and is expected to experience significant growth over the forecast period.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market Structure & Competitive Landscape
The Pharmaceutical CDMO market is characterized by a moderately consolidated structure, with a few large players holding significant market share alongside numerous smaller, specialized firms. Market concentration is expected to increase further due to ongoing mergers and acquisitions (M&A) activity. The xx Million market displays a Herfindahl-Hirschman Index (HHI) of xx in 2025, indicating a moderately concentrated market. Innovation is a key driver, fueled by advancements in drug delivery technologies, personalized medicine, and cell and gene therapies. Regulatory changes, including stricter guidelines on Good Manufacturing Practices (GMP) and data integrity, significantly impact market dynamics, favoring companies with robust compliance programs. Substitute products are limited, as CDMO services often offer specialized expertise and economies of scale unavailable to in-house development. End-user segmentation is primarily driven by pharmaceutical and biotechnology companies of varying sizes, ranging from large multinational corporations to smaller biotech startups. M&A activity has been substantial in recent years, with a volume of xx deals recorded between 2019 and 2024, primarily driven by the desire to expand service offerings, geographic reach, and technological capabilities.
- Market Concentration: Moderately consolidated, with an HHI of xx in 2025.
- Innovation Drivers: Advancements in drug delivery, personalized medicine, cell & gene therapies.
- Regulatory Impacts: Stricter GMP and data integrity guidelines favor compliant companies.
- End-User Segmentation: Pharmaceutical and biotechnology companies of various sizes.
- M&A Trends: Significant activity (xx deals from 2019-2024) driven by expansion and technological advancements.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market Trends & Opportunities
The global Pharmaceutical CDMO market is experiencing robust growth, with a projected Compound Annual Growth Rate (CAGR) of xx% from 2025 to 2033, driven by several key factors. The increasing outsourcing of drug development and manufacturing activities by pharmaceutical companies is a major contributor. This trend is fueled by cost optimization, access to specialized expertise, and faster time-to-market. Technological advancements, such as the adoption of automation, artificial intelligence (AI), and advanced analytical techniques are transforming the CDMO landscape, improving efficiency and enhancing product quality. Growing demand for specialized services, including high-potency APIs (HPAPIs) and complex formulations, presents significant opportunities for specialized CDMOs. The market penetration rate for CDMO services in the pharmaceutical industry is estimated at xx% in 2025, projected to reach xx% by 2033. Competitive dynamics are shaped by factors such as pricing strategies, technological capabilities, and regulatory compliance. The emergence of new technologies and business models, such as cell and gene therapy manufacturing, is reshaping the competitive landscape. Further growth will be propelled by rising prevalence of chronic diseases, an aging global population, and an increasing focus on innovative drug development.
Dominant Markets & Segments in Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
The North American region currently dominates the global Pharmaceutical CDMO market, driven by strong research and development activity, a large number of pharmaceutical and biotechnology companies, and robust regulatory frameworks. Within this region, the United States is a major market. However, emerging markets in Asia-Pacific are showing rapid growth, fueled by increasing healthcare spending, government support for the pharmaceutical industry, and a burgeoning middle class. The solid dose formulation segment, specifically tablets, holds the largest share due to high volume manufacturing and relatively simpler production processes. However, the Injectable Dose Formulation and HPAPI segments are experiencing faster growth rates due to the rising demand for complex and specialized drug products.
Key Growth Drivers by Segment:
- North America: Strong R&D activity, large pharmaceutical presence, robust regulations.
- Asia-Pacific: Increasing healthcare spending, government support, burgeoning middle class.
- Solid Dose Formulation (Tablets): High volume manufacturing, simpler processes.
- Injectable Dose Formulation & HPAPI: Rising demand for complex and specialized drugs.
- By Research Phase CRO Segment: Phase III clinical trials currently dominate, reflecting the stage of drug development requiring extensive testing and manufacturing capacity. However, growth is anticipated across all phases, particularly Pre-clinical and Phase I due to growing investments in early-stage drug discovery.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Product Analysis
Recent advancements in process analytical technology (PAT) and continuous manufacturing are transforming CDMO product offerings. These innovations improve process control, reduce manufacturing costs, and accelerate product development timelines. CDMOs are increasingly offering integrated services, encompassing all aspects of drug development and manufacturing, from API synthesis to final packaging. This integrated approach enhances efficiency and reduces coordination challenges for clients. The market fit for these advanced services is high as clients seek to optimize their drug development and manufacturing processes.
Key Drivers, Barriers & Challenges in Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
Key Drivers:
Technological advancements (automation, AI, PAT), increasing outsourcing by pharmaceutical companies, growing demand for specialized services (HPAPIs, complex formulations), rising healthcare spending, and government support for pharmaceutical innovation. For example, the increasing adoption of AI-powered drug discovery platforms is accelerating the speed and efficiency of drug development, creating higher demand for CDMO services specializing in these novel technologies.
Key Challenges & Restraints:
Supply chain disruptions can cause significant delays and cost increases, impacting production timelines and profitability. Stringent regulatory requirements and compliance costs are major hurdles for CDMOs, particularly in navigating changing guidelines across different jurisdictions. Intense competition among CDMOs drives down pricing and margins, necessitating operational efficiency. For instance, a 10% increase in raw material prices can decrease CDMO profit margins by xx%, demonstrating supply chain vulnerability.
Growth Drivers in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market
The CDMO market is primarily driven by the increasing outsourcing of drug development and manufacturing. Pharmaceutical companies outsource to gain access to specialized expertise, reduce operational costs, and accelerate their time to market. Technological innovations such as AI and automation are significantly enhancing productivity and efficiency in CDMO operations. Furthermore, favorable regulatory frameworks and increased investments in healthcare infrastructure in many regions are catalyzing market expansion.
Challenges Impacting Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Growth
Regulatory hurdles, particularly concerning GMP compliance and data integrity, pose significant challenges. Supply chain vulnerabilities, including raw material shortages and geopolitical instability, can cause production delays. The fiercely competitive landscape with many players vying for contracts can lead to price pressure and reduced profit margins.
Key Players Shaping the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market
- LSK Global Pharma Service Co Ltd
- Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc)
- Hangzhou Tigermed Consulting Co Ltd
- Famar SA
- PAREXEL International Corporation
- CMIC Holdings Co Ltd
- PRA Health Sciences Inc (Icon PLC)
- Lonza Group
- Pfizer CentreSource
- SGS Life Science Services SA
- Samsung Bioepis Co Ltd
- Jubilant Pharmova Ltd
- WuXi AppTec Inc
- Tesa Labtec GmbH (TESA SE)
- Patheon Inc (Thermo Fisher Scientific Inc)
- Syneos Health Inc
- IQVIA Holdings Inc
- ARX LLC
- Tapemark
- Catalent Inc
- Boehringer Ingelheim Group
- Novotech Pty Ltd
- LabCorp Drug Development
- Aenova Holding GmbH
- Recipharm AB
- Sagimet Biosciences (3V Biosciences Inc)
- Baxter Biopharma Solutions (Baxter International Inc)
- Quanticate Ltd
Significant Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Industry Milestones
- January 2024: FAMAR and Lavipharm announce a collaboration, strengthening the European CDMO landscape.
- January 2024: Pluri launches pluriCDMO, expanding cell therapy manufacturing capacity.
- October 2023: IQVIA and Argenx collaborate on innovative pharmacovigilance solutions.
Future Outlook for Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Market
The Pharmaceutical CDMO market is poised for sustained growth, driven by ongoing technological advancements, increasing outsourcing trends, and expansion into emerging markets. Strategic partnerships and acquisitions will continue to reshape the competitive landscape, with a focus on integrating services and expanding technological capabilities. The market's growth potential is significant, driven by the increasing demand for specialized services and the continued innovation within the pharmaceutical and biotechnology industries.
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Segmentation
-
1. Service Type CMO Segment
-
1.1. Active P
- 1.1.1. Small Molecule
- 1.1.2. Large Molecule
- 1.1.3. High Potency (HPAPI)
-
1.2. Finished
-
1.2.1. Solid Dose Formulation
- 1.2.1.1. Tablets
- 1.2.1.2. Others (Capsules, Powders, etc.)
- 1.2.2. Liquid Dose Formulation
- 1.2.3. Injectable Dose Formulation
-
1.2.1. Solid Dose Formulation
- 1.3. Secondary Packaging
-
1.1. Active P
-
2. Research Phase CRO Segment
- 2.1. Pre-clinical
- 2.2. Phase I
- 2.3. Phase II
- 2.4. Phase III
- 2.5. Phase IV
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
-
2. Europe
- 2.1. United Kingdom
- 2.2. Germany
- 2.3. France
- 2.4. Italy
-
3. Asia
- 3.1. China
- 3.2. India
- 3.3. Japan
- 3.4. Australia and New Zealand
-
4. Latin America
- 4.1. Brazil
- 4.2. Mexico
- 4.3. Argentina
-
5. Middle East and Africa
- 5.1. United Arab Emirates
- 5.2. Saudi Arabia
- 5.3. South Africa
- 6. North America
- 7. Europe
- 8. Asia
- 9. Australia and New Zealand
- 10. Latin America
- 11. Middle East and Africa
-Market.png)
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Regional Market Share

Geographic Coverage of Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market
Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.41% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Outsourcing Volume by Big Pharmaceutical Companies; Advent of CDMO Model into the Market; Increasing Investment in R&D
- 3.3. Market Restrains
- 3.3.1. Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs
- 3.4. Market Trends
- 3.4.1. Increasing Investment in R&D Drives the Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 5.1.1. Active P
- 5.1.1.1. Small Molecule
- 5.1.1.2. Large Molecule
- 5.1.1.3. High Potency (HPAPI)
- 5.1.2. Finished
- 5.1.2.1. Solid Dose Formulation
- 5.1.2.1.1. Tablets
- 5.1.2.1.2. Others (Capsules, Powders, etc.)
- 5.1.2.2. Liquid Dose Formulation
- 5.1.2.3. Injectable Dose Formulation
- 5.1.2.1. Solid Dose Formulation
- 5.1.3. Secondary Packaging
- 5.1.1. Active P
- 5.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 5.2.1. Pre-clinical
- 5.2.2. Phase I
- 5.2.3. Phase II
- 5.2.4. Phase III
- 5.2.5. Phase IV
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia
- 5.3.4. Latin America
- 5.3.5. Middle East and Africa
- 5.3.6. North America
- 5.3.7. Europe
- 5.3.8. Asia
- 5.3.9. Australia and New Zealand
- 5.3.10. Latin America
- 5.3.11. Middle East and Africa
- 5.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 6. North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 6.1.1. Active P
- 6.1.1.1. Small Molecule
- 6.1.1.2. Large Molecule
- 6.1.1.3. High Potency (HPAPI)
- 6.1.2. Finished
- 6.1.2.1. Solid Dose Formulation
- 6.1.2.1.1. Tablets
- 6.1.2.1.2. Others (Capsules, Powders, etc.)
- 6.1.2.2. Liquid Dose Formulation
- 6.1.2.3. Injectable Dose Formulation
- 6.1.2.1. Solid Dose Formulation
- 6.1.3. Secondary Packaging
- 6.1.1. Active P
- 6.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 6.2.1. Pre-clinical
- 6.2.2. Phase I
- 6.2.3. Phase II
- 6.2.4. Phase III
- 6.2.5. Phase IV
- 6.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 7. Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 7.1.1. Active P
- 7.1.1.1. Small Molecule
- 7.1.1.2. Large Molecule
- 7.1.1.3. High Potency (HPAPI)
- 7.1.2. Finished
- 7.1.2.1. Solid Dose Formulation
- 7.1.2.1.1. Tablets
- 7.1.2.1.2. Others (Capsules, Powders, etc.)
- 7.1.2.2. Liquid Dose Formulation
- 7.1.2.3. Injectable Dose Formulation
- 7.1.2.1. Solid Dose Formulation
- 7.1.3. Secondary Packaging
- 7.1.1. Active P
- 7.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 7.2.1. Pre-clinical
- 7.2.2. Phase I
- 7.2.3. Phase II
- 7.2.4. Phase III
- 7.2.5. Phase IV
- 7.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 8. Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 8.1.1. Active P
- 8.1.1.1. Small Molecule
- 8.1.1.2. Large Molecule
- 8.1.1.3. High Potency (HPAPI)
- 8.1.2. Finished
- 8.1.2.1. Solid Dose Formulation
- 8.1.2.1.1. Tablets
- 8.1.2.1.2. Others (Capsules, Powders, etc.)
- 8.1.2.2. Liquid Dose Formulation
- 8.1.2.3. Injectable Dose Formulation
- 8.1.2.1. Solid Dose Formulation
- 8.1.3. Secondary Packaging
- 8.1.1. Active P
- 8.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 8.2.1. Pre-clinical
- 8.2.2. Phase I
- 8.2.3. Phase II
- 8.2.4. Phase III
- 8.2.5. Phase IV
- 8.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 9. Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 9.1.1. Active P
- 9.1.1.1. Small Molecule
- 9.1.1.2. Large Molecule
- 9.1.1.3. High Potency (HPAPI)
- 9.1.2. Finished
- 9.1.2.1. Solid Dose Formulation
- 9.1.2.1.1. Tablets
- 9.1.2.1.2. Others (Capsules, Powders, etc.)
- 9.1.2.2. Liquid Dose Formulation
- 9.1.2.3. Injectable Dose Formulation
- 9.1.2.1. Solid Dose Formulation
- 9.1.3. Secondary Packaging
- 9.1.1. Active P
- 9.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 9.2.1. Pre-clinical
- 9.2.2. Phase I
- 9.2.3. Phase II
- 9.2.4. Phase III
- 9.2.5. Phase IV
- 9.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 10. Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 10.1.1. Active P
- 10.1.1.1. Small Molecule
- 10.1.1.2. Large Molecule
- 10.1.1.3. High Potency (HPAPI)
- 10.1.2. Finished
- 10.1.2.1. Solid Dose Formulation
- 10.1.2.1.1. Tablets
- 10.1.2.1.2. Others (Capsules, Powders, etc.)
- 10.1.2.2. Liquid Dose Formulation
- 10.1.2.3. Injectable Dose Formulation
- 10.1.2.1. Solid Dose Formulation
- 10.1.3. Secondary Packaging
- 10.1.1. Active P
- 10.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 10.2.1. Pre-clinical
- 10.2.2. Phase I
- 10.2.3. Phase II
- 10.2.4. Phase III
- 10.2.5. Phase IV
- 10.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 11. North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 11.1.1. Active P
- 11.1.1.1. Small Molecule
- 11.1.1.2. Large Molecule
- 11.1.1.3. High Potency (HPAPI)
- 11.1.2. Finished
- 11.1.2.1. Solid Dose Formulation
- 11.1.2.1.1. Tablets
- 11.1.2.1.2. Others (Capsules, Powders, etc.)
- 11.1.2.2. Liquid Dose Formulation
- 11.1.2.3. Injectable Dose Formulation
- 11.1.2.1. Solid Dose Formulation
- 11.1.3. Secondary Packaging
- 11.1.1. Active P
- 11.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 11.2.1. Pre-clinical
- 11.2.2. Phase I
- 11.2.3. Phase II
- 11.2.4. Phase III
- 11.2.5. Phase IV
- 11.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 12. Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 12.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 12.1.1. Active P
- 12.1.1.1. Small Molecule
- 12.1.1.2. Large Molecule
- 12.1.1.3. High Potency (HPAPI)
- 12.1.2. Finished
- 12.1.2.1. Solid Dose Formulation
- 12.1.2.1.1. Tablets
- 12.1.2.1.2. Others (Capsules, Powders, etc.)
- 12.1.2.2. Liquid Dose Formulation
- 12.1.2.3. Injectable Dose Formulation
- 12.1.2.1. Solid Dose Formulation
- 12.1.3. Secondary Packaging
- 12.1.1. Active P
- 12.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 12.2.1. Pre-clinical
- 12.2.2. Phase I
- 12.2.3. Phase II
- 12.2.4. Phase III
- 12.2.5. Phase IV
- 12.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 13. Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 13.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 13.1.1. Active P
- 13.1.1.1. Small Molecule
- 13.1.1.2. Large Molecule
- 13.1.1.3. High Potency (HPAPI)
- 13.1.2. Finished
- 13.1.2.1. Solid Dose Formulation
- 13.1.2.1.1. Tablets
- 13.1.2.1.2. Others (Capsules, Powders, etc.)
- 13.1.2.2. Liquid Dose Formulation
- 13.1.2.3. Injectable Dose Formulation
- 13.1.2.1. Solid Dose Formulation
- 13.1.3. Secondary Packaging
- 13.1.1. Active P
- 13.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 13.2.1. Pre-clinical
- 13.2.2. Phase I
- 13.2.3. Phase II
- 13.2.4. Phase III
- 13.2.5. Phase IV
- 13.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 14. Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 14.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 14.1.1. Active P
- 14.1.1.1. Small Molecule
- 14.1.1.2. Large Molecule
- 14.1.1.3. High Potency (HPAPI)
- 14.1.2. Finished
- 14.1.2.1. Solid Dose Formulation
- 14.1.2.1.1. Tablets
- 14.1.2.1.2. Others (Capsules, Powders, etc.)
- 14.1.2.2. Liquid Dose Formulation
- 14.1.2.3. Injectable Dose Formulation
- 14.1.2.1. Solid Dose Formulation
- 14.1.3. Secondary Packaging
- 14.1.1. Active P
- 14.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 14.2.1. Pre-clinical
- 14.2.2. Phase I
- 14.2.3. Phase II
- 14.2.4. Phase III
- 14.2.5. Phase IV
- 14.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 15. Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 15.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 15.1.1. Active P
- 15.1.1.1. Small Molecule
- 15.1.1.2. Large Molecule
- 15.1.1.3. High Potency (HPAPI)
- 15.1.2. Finished
- 15.1.2.1. Solid Dose Formulation
- 15.1.2.1.1. Tablets
- 15.1.2.1.2. Others (Capsules, Powders, etc.)
- 15.1.2.2. Liquid Dose Formulation
- 15.1.2.3. Injectable Dose Formulation
- 15.1.2.1. Solid Dose Formulation
- 15.1.3. Secondary Packaging
- 15.1.1. Active P
- 15.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 15.2.1. Pre-clinical
- 15.2.2. Phase I
- 15.2.3. Phase II
- 15.2.4. Phase III
- 15.2.5. Phase IV
- 15.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 16. Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Analysis, Insights and Forecast, 2020-2032
- 16.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 16.1.1. Active P
- 16.1.1.1. Small Molecule
- 16.1.1.2. Large Molecule
- 16.1.1.3. High Potency (HPAPI)
- 16.1.2. Finished
- 16.1.2.1. Solid Dose Formulation
- 16.1.2.1.1. Tablets
- 16.1.2.1.2. Others (Capsules, Powders, etc.)
- 16.1.2.2. Liquid Dose Formulation
- 16.1.2.3. Injectable Dose Formulation
- 16.1.2.1. Solid Dose Formulation
- 16.1.3. Secondary Packaging
- 16.1.1. Active P
- 16.2. Market Analysis, Insights and Forecast - by Research Phase CRO Segment
- 16.2.1. Pre-clinical
- 16.2.2. Phase I
- 16.2.3. Phase II
- 16.2.4. Phase III
- 16.2.5. Phase IV
- 16.1. Market Analysis, Insights and Forecast - by Service Type CMO Segment
- 17. Competitive Analysis
- 17.1. Global Market Share Analysis 2025
- 17.2. Company Profiles
- 17.2.1 LSK Global Pharma Service Co Ltd
- 17.2.1.1. Overview
- 17.2.1.2. Products
- 17.2.1.3. SWOT Analysis
- 17.2.1.4. Recent Developments
- 17.2.1.5. Financials (Based on Availability)
- 17.2.2 Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc )
- 17.2.2.1. Overview
- 17.2.2.2. Products
- 17.2.2.3. SWOT Analysis
- 17.2.2.4. Recent Developments
- 17.2.2.5. Financials (Based on Availability)
- 17.2.3 Hangzhou Tigermed Consulting Co Ltd
- 17.2.3.1. Overview
- 17.2.3.2. Products
- 17.2.3.3. SWOT Analysis
- 17.2.3.4. Recent Developments
- 17.2.3.5. Financials (Based on Availability)
- 17.2.4 Famar SA
- 17.2.4.1. Overview
- 17.2.4.2. Products
- 17.2.4.3. SWOT Analysis
- 17.2.4.4. Recent Developments
- 17.2.4.5. Financials (Based on Availability)
- 17.2.5 PAREXEL International Corporation
- 17.2.5.1. Overview
- 17.2.5.2. Products
- 17.2.5.3. SWOT Analysis
- 17.2.5.4. Recent Developments
- 17.2.5.5. Financials (Based on Availability)
- 17.2.6 CMIC Holdings Co Ltd
- 17.2.6.1. Overview
- 17.2.6.2. Products
- 17.2.6.3. SWOT Analysis
- 17.2.6.4. Recent Developments
- 17.2.6.5. Financials (Based on Availability)
- 17.2.7 PRA Health Sciences Inc (Icon PLC)
- 17.2.7.1. Overview
- 17.2.7.2. Products
- 17.2.7.3. SWOT Analysis
- 17.2.7.4. Recent Developments
- 17.2.7.5. Financials (Based on Availability)
- 17.2.8 Lonza Group
- 17.2.8.1. Overview
- 17.2.8.2. Products
- 17.2.8.3. SWOT Analysis
- 17.2.8.4. Recent Developments
- 17.2.8.5. Financials (Based on Availability)
- 17.2.9 Pfizer CentreSource
- 17.2.9.1. Overview
- 17.2.9.2. Products
- 17.2.9.3. SWOT Analysis
- 17.2.9.4. Recent Developments
- 17.2.9.5. Financials (Based on Availability)
- 17.2.10 SGS Life Science Services SA
- 17.2.10.1. Overview
- 17.2.10.2. Products
- 17.2.10.3. SWOT Analysis
- 17.2.10.4. Recent Developments
- 17.2.10.5. Financials (Based on Availability)
- 17.2.11 Samsung Bioepis Co Ltd
- 17.2.11.1. Overview
- 17.2.11.2. Products
- 17.2.11.3. SWOT Analysis
- 17.2.11.4. Recent Developments
- 17.2.11.5. Financials (Based on Availability)
- 17.2.12 Jubilant Pharmova Ltd
- 17.2.12.1. Overview
- 17.2.12.2. Products
- 17.2.12.3. SWOT Analysis
- 17.2.12.4. Recent Developments
- 17.2.12.5. Financials (Based on Availability)
- 17.2.13 WuXi AppTec Inc
- 17.2.13.1. Overview
- 17.2.13.2. Products
- 17.2.13.3. SWOT Analysis
- 17.2.13.4. Recent Developments
- 17.2.13.5. Financials (Based on Availability)
- 17.2.14 Tesa Labtec GmbH (TESA SE)
- 17.2.14.1. Overview
- 17.2.14.2. Products
- 17.2.14.3. SWOT Analysis
- 17.2.14.4. Recent Developments
- 17.2.14.5. Financials (Based on Availability)
- 17.2.15 Patheon Inc (Thermo Fisher Scientific Inc )
- 17.2.15.1. Overview
- 17.2.15.2. Products
- 17.2.15.3. SWOT Analysis
- 17.2.15.4. Recent Developments
- 17.2.15.5. Financials (Based on Availability)
- 17.2.16 Syneos Health Inc
- 17.2.16.1. Overview
- 17.2.16.2. Products
- 17.2.16.3. SWOT Analysis
- 17.2.16.4. Recent Developments
- 17.2.16.5. Financials (Based on Availability)
- 17.2.17 IQVIA Holdings Inc
- 17.2.17.1. Overview
- 17.2.17.2. Products
- 17.2.17.3. SWOT Analysis
- 17.2.17.4. Recent Developments
- 17.2.17.5. Financials (Based on Availability)
- 17.2.18 ARX LLC
- 17.2.18.1. Overview
- 17.2.18.2. Products
- 17.2.18.3. SWOT Analysis
- 17.2.18.4. Recent Developments
- 17.2.18.5. Financials (Based on Availability)
- 17.2.19 Tapemark
- 17.2.19.1. Overview
- 17.2.19.2. Products
- 17.2.19.3. SWOT Analysis
- 17.2.19.4. Recent Developments
- 17.2.19.5. Financials (Based on Availability)
- 17.2.20 Catalent Inc
- 17.2.20.1. Overview
- 17.2.20.2. Products
- 17.2.20.3. SWOT Analysis
- 17.2.20.4. Recent Developments
- 17.2.20.5. Financials (Based on Availability)
- 17.2.21 Boehringer Ingelheim Group
- 17.2.21.1. Overview
- 17.2.21.2. Products
- 17.2.21.3. SWOT Analysis
- 17.2.21.4. Recent Developments
- 17.2.21.5. Financials (Based on Availability)
- 17.2.22 Novotech Pty Ltd
- 17.2.22.1. Overview
- 17.2.22.2. Products
- 17.2.22.3. SWOT Analysis
- 17.2.22.4. Recent Developments
- 17.2.22.5. Financials (Based on Availability)
- 17.2.23 LabCorp Drug Development
- 17.2.23.1. Overview
- 17.2.23.2. Products
- 17.2.23.3. SWOT Analysis
- 17.2.23.4. Recent Developments
- 17.2.23.5. Financials (Based on Availability)
- 17.2.24 Aenova Holding GmbH
- 17.2.24.1. Overview
- 17.2.24.2. Products
- 17.2.24.3. SWOT Analysis
- 17.2.24.4. Recent Developments
- 17.2.24.5. Financials (Based on Availability)
- 17.2.25 Recipharm AB
- 17.2.25.1. Overview
- 17.2.25.2. Products
- 17.2.25.3. SWOT Analysis
- 17.2.25.4. Recent Developments
- 17.2.25.5. Financials (Based on Availability)
- 17.2.26 Sagimet Biosciences (3V Biosciences Inc )*List Not Exhaustive
- 17.2.26.1. Overview
- 17.2.26.2. Products
- 17.2.26.3. SWOT Analysis
- 17.2.26.4. Recent Developments
- 17.2.26.5. Financials (Based on Availability)
- 17.2.27 Baxter Biopharma Solutions (Baxter International Inc )
- 17.2.27.1. Overview
- 17.2.27.2. Products
- 17.2.27.3. SWOT Analysis
- 17.2.27.4. Recent Developments
- 17.2.27.5. Financials (Based on Availability)
- 17.2.28 Quanticate Ltd
- 17.2.28.1. Overview
- 17.2.28.2. Products
- 17.2.28.3. SWOT Analysis
- 17.2.28.4. Recent Developments
- 17.2.28.5. Financials (Based on Availability)
- 17.2.1 LSK Global Pharma Service Co Ltd
List of Figures
- Figure 1: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 3: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 4: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 5: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 6: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 7: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 8: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 9: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 10: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 11: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 12: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 13: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 15: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 16: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 17: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 18: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 19: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 20: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 21: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 22: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 23: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 24: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 25: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 27: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 28: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 29: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 30: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 31: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 32: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 33: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 34: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 35: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 36: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 37: North America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 38: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 39: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 40: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 41: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 42: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 43: Europe Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 44: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 45: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 46: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 47: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 48: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 49: Asia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 50: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 51: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 52: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 53: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 54: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 55: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 56: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 57: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 58: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 59: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 60: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 61: Latin America Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
- Figure 62: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Service Type CMO Segment 2025 & 2033
- Figure 63: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Service Type CMO Segment 2025 & 2033
- Figure 64: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Research Phase CRO Segment 2025 & 2033
- Figure 65: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Research Phase CRO Segment 2025 & 2033
- Figure 66: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million), by Country 2025 & 2033
- Figure 67: Middle East and Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 2: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 3: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Region 2020 & 2033
- Table 4: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 5: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 6: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 7: United States Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 8: Canada Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 9: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 10: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 11: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 12: United Kingdom Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 13: Germany Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 14: France Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 15: Italy Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 16: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 17: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 18: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 19: China Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: India Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 21: Japan Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 22: Australia and New Zealand Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 23: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 24: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 25: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 26: Brazil Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 27: Mexico Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 28: Argentina Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 29: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 30: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 31: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 32: United Arab Emirates Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 33: Saudi Arabia Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 34: South Africa Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 35: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 36: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 37: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 38: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 39: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 40: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 41: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 42: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 43: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 44: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 45: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 46: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 47: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 48: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 49: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
- Table 50: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Service Type CMO Segment 2020 & 2033
- Table 51: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Research Phase CRO Segment 2020 & 2033
- Table 52: Global Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market Revenue Million Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
The projected CAGR is approximately 6.41%.
2. Which companies are prominent players in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
Key companies in the market include LSK Global Pharma Service Co Ltd, Pharmaceutical Product Development LLC (Thermo Fisher Scientific Inc ), Hangzhou Tigermed Consulting Co Ltd, Famar SA, PAREXEL International Corporation, CMIC Holdings Co Ltd, PRA Health Sciences Inc (Icon PLC), Lonza Group, Pfizer CentreSource, SGS Life Science Services SA, Samsung Bioepis Co Ltd, Jubilant Pharmova Ltd, WuXi AppTec Inc, Tesa Labtec GmbH (TESA SE), Patheon Inc (Thermo Fisher Scientific Inc ), Syneos Health Inc, IQVIA Holdings Inc, ARX LLC, Tapemark, Catalent Inc, Boehringer Ingelheim Group, Novotech Pty Ltd, LabCorp Drug Development, Aenova Holding GmbH, Recipharm AB, Sagimet Biosciences (3V Biosciences Inc )*List Not Exhaustive, Baxter Biopharma Solutions (Baxter International Inc ), Quanticate Ltd.
3. What are the main segments of the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
The market segments include Service Type CMO Segment, Research Phase CRO Segment.
4. Can you provide details about the market size?
The market size is estimated to be USD 243.29 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Outsourcing Volume by Big Pharmaceutical Companies; Advent of CDMO Model into the Market; Increasing Investment in R&D.
6. What are the notable trends driving market growth?
Increasing Investment in R&D Drives the Market.
7. Are there any restraints impacting market growth?
Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs.
8. Can you provide examples of recent developments in the market?
January 2024 - A new collaboration was announced jointly by FAMAR and Lavipharm, two leading pharmaceutical companies. FAMAR is a leading provider of development and manufacturing services for pharmaceutical and cosmetic products (CDMO) and one of the major CDMO players in Europe. Lavipharm is a research and development (R&D) company that manufactures, imports, sells, and distributes pharmaceuticals and healthcare products in Greece.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market?
To stay informed about further developments, trends, and reports in the Pharmaceutical Contract Development and Manufacturing Organization (CDMO) Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
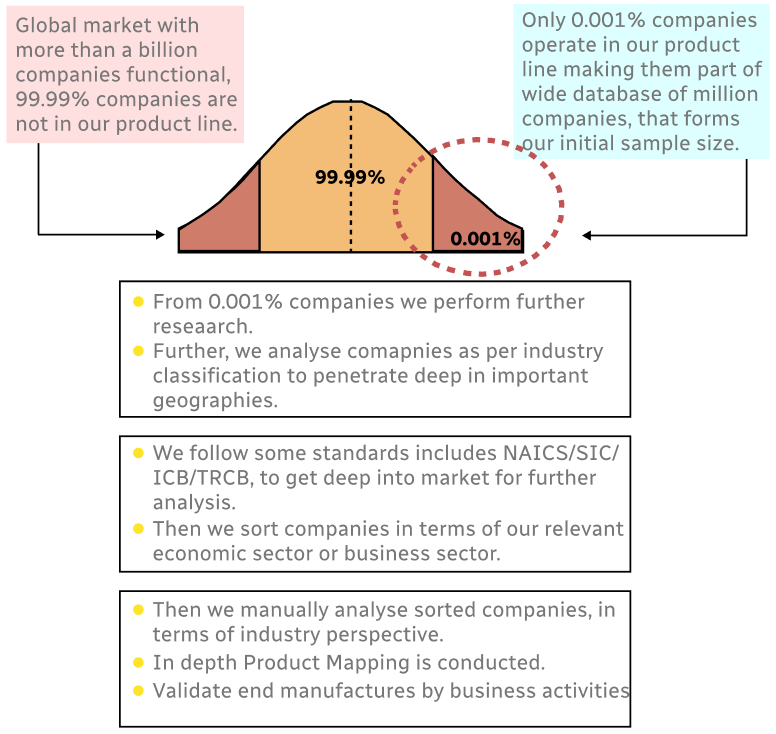
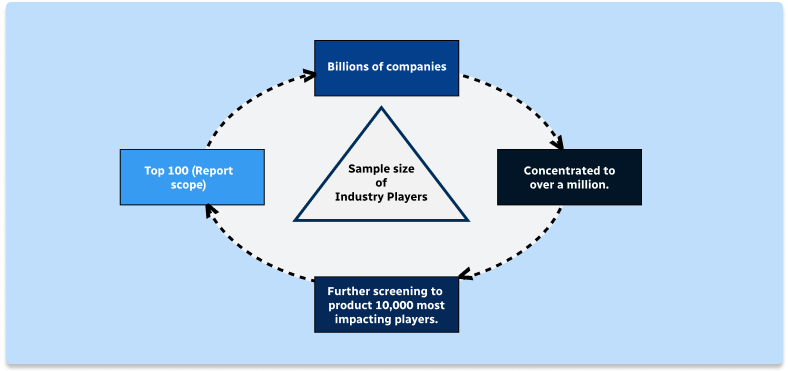
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
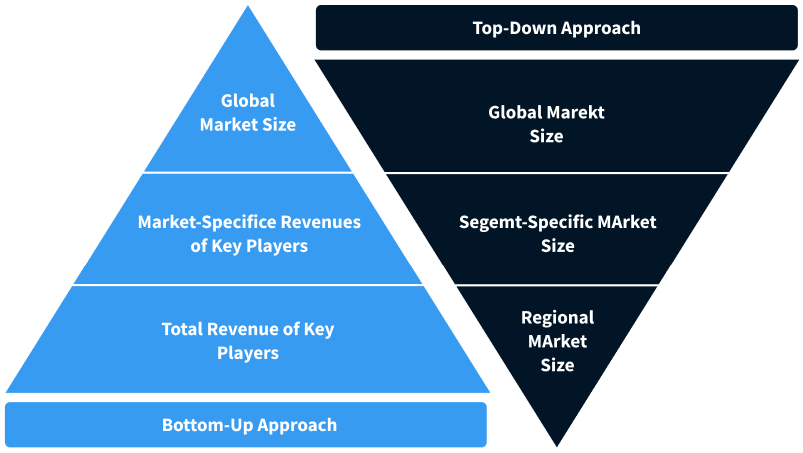
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


